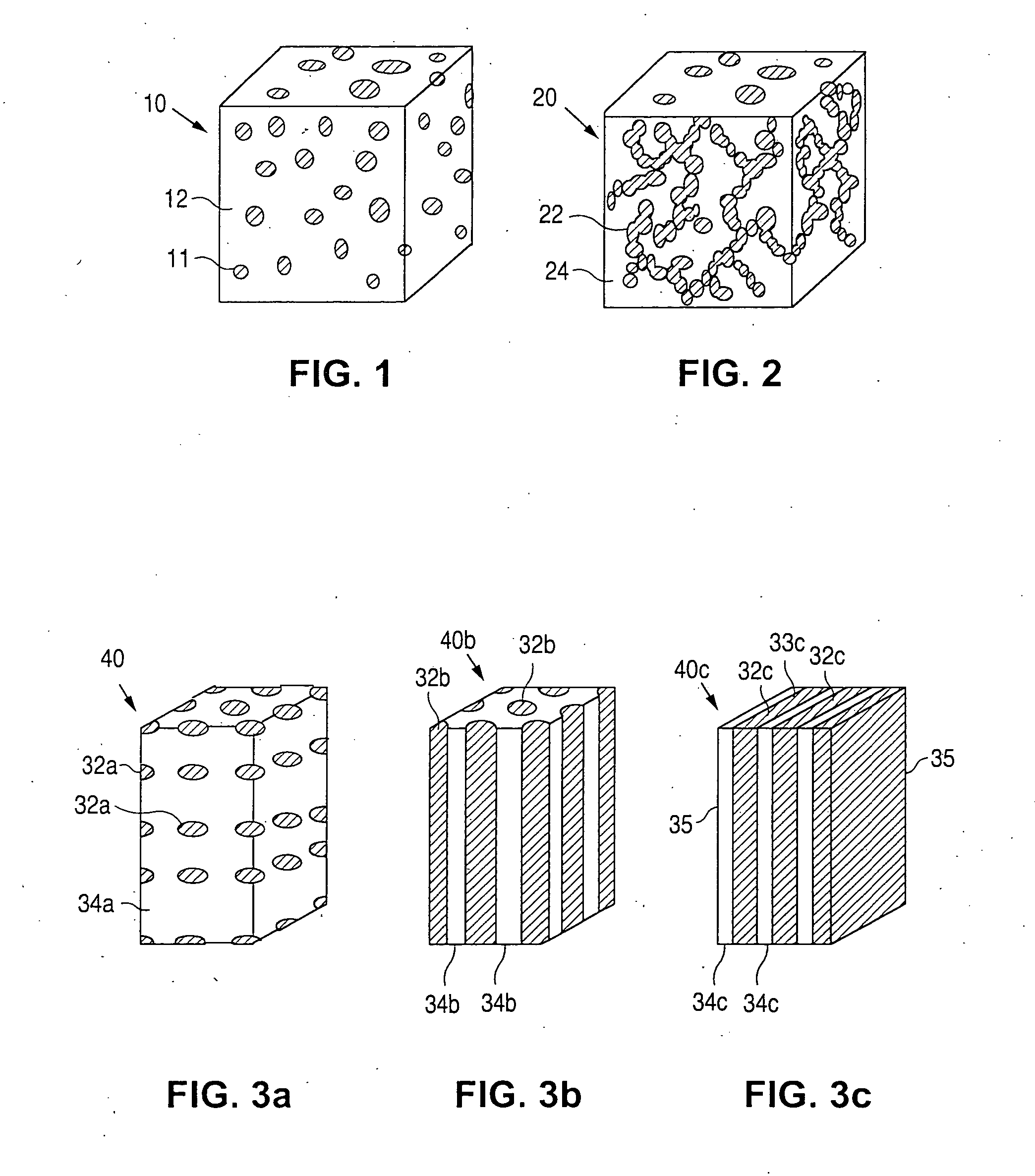
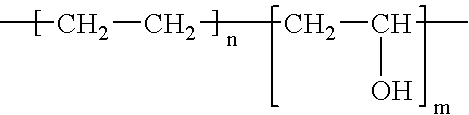Phase-separated polymer coatings
a phase separation and polymer coating technology, applied in the direction of powder delivery, medical preparations, microcapsules, etc., can solve the problems of difficult to prevent a rapid, uncontrolled release of drugs, and the troublesome burst effect, and achieve the effect of decreasing the rate of drug releas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0037] One exemplary composition that produces the drug release morphology of FIG. 3c includes an EVAL polymer with 66 weight percent ethylene groups, 43.32 weight percent vinyl alcohol functionalities and 0.68 weight percent vinyl ether groups. The weight percent refers to the percent of the total drug release system weight. The vinyl ether groups were functionalized with PEO-isocyanate, which forms a urethane linkage, using groups that have a molecular weight of a side group of 3200 g / mol. The side groups comprise 33 weight percent of the total EVAL / PEO polymer. The composition of the PEO-isocyanate blend is 75 weight percent functionalized EVAL and 25 weight percent drug. This composition gives rise to a 50 weight percent hard, bulk phase and a 50 weight percent drug / PEO side chain phase. The final structure is a lamellar structure.
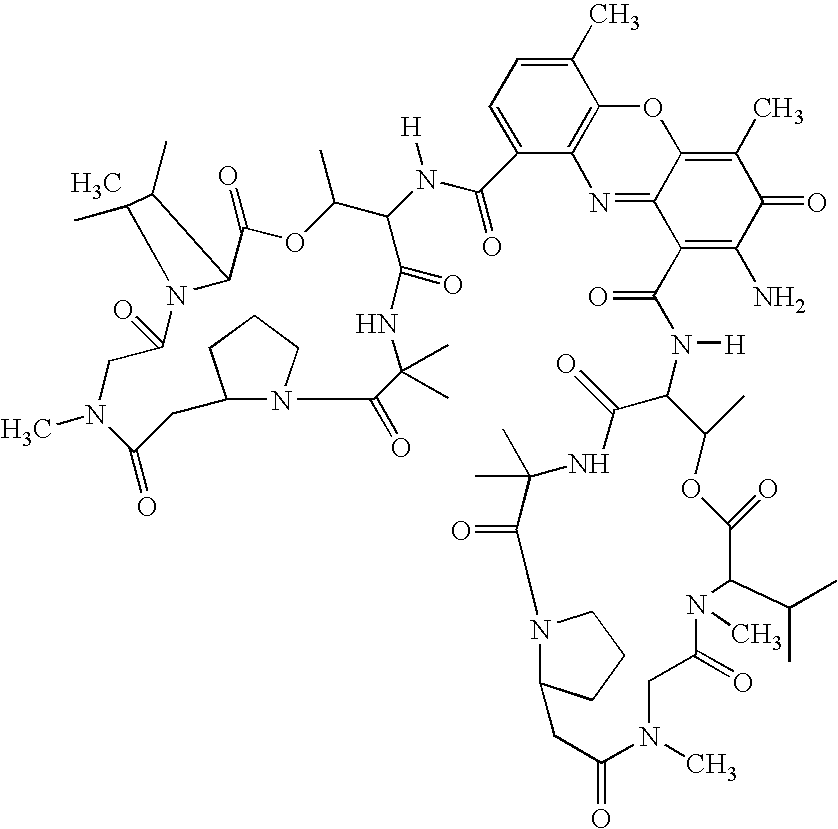
[0038] The chemical reaction is as follows:
[0039] (x)=66 weight %; (y)=44 weight %. M is approximately equal to 70 units. Molecular weight is appro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com