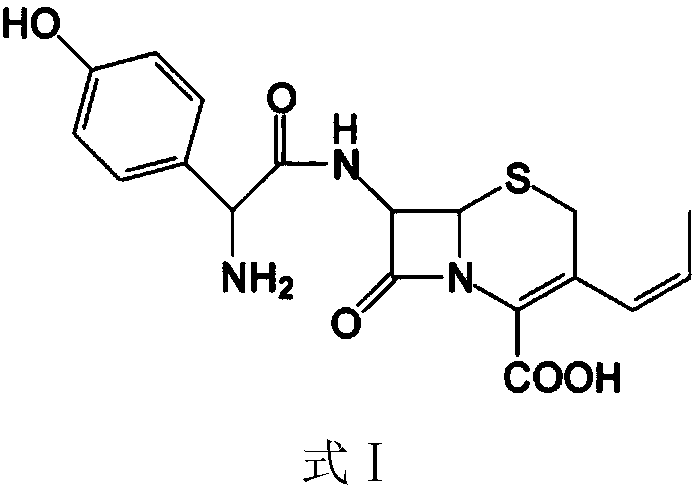
Preparation method of cefprozil
A technology of cefprozil and 7-ACA, which is applied in the field of medicine, can solve the problems of affecting the ability of enzymes to catalyze synthesis, cannot achieve industrial production, and the product purity is not high, and achieve the effect of mild conditions, easy scale-up, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
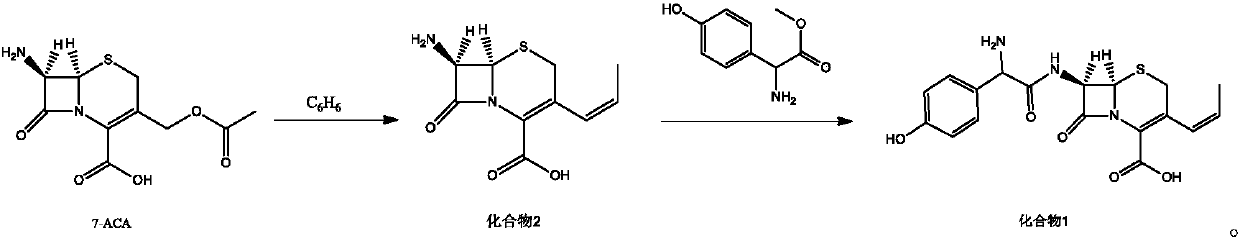
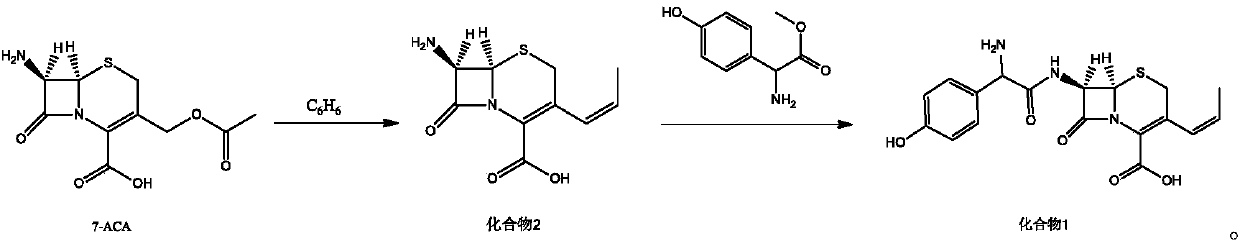
[0018] Embodiment 1: the synthesis of compound 2
[0019] Under nitrogen protection, 100mL of benzene, 40mmol of 7-ACA, 48mmol of hexamethyldisilazane, and 0.5mmol of pyridine were successively added to the reaction flask, stirred and heated to reflux for 2 hours, cooled to 0°C, and three Methyl iodosilane (TMSI) 44mmol, keep warm for 1h, add 38mmol of trifluoroethoxy phosphate, continue the reaction for 1h, add 36mmol of sodium hexamethyldisilazide, 60mmol of 15-crown-5, heat and reflux for 30min, cool to At room temperature, 36 mmol of acetaldehyde was added and reacted at room temperature for 6 h. After the reaction is complete, add 50mL of water to the reaction solution, stir, collect the organic phase, extract with 25mL of 3mol / L hydrochloric acid in 3 times, combine the water layers, wash with 50mL of dichloromethane, separate the organic layer, and wash the obtained aqueous solution with 20% sodium hydroxide The pH of the solution was adjusted to 2.5, and a solid preci...
Embodiment 2
[0020] Embodiment 2: the synthesis of compound 2
[0021] Under the protection of nitrogen, add 100mL of benzene, 40mmol of 7-ACA, 40mmol of hexamethyldisilazane and 0.5mmol of pyridine in turn to the reaction flask, stir and heat up to reflux for 2h, cool the reaction solution to 0°C, drop three Methyl iodosilane (TMSI) 40mmol, keep warm for 1h, add 32mmol of trifluoroethoxy phosphate, continue the reaction for 1h, add 32mmol of sodium hexamethyldisilazide, 40mmol of 15-crown-5, heat and reflux for 30min, cool to At room temperature, 32 mmol of acetaldehyde was added and reacted at room temperature for 6 h. After the reaction is complete, add 50mL of water to the reaction solution, stir, collect the organic phase, extract with 25mL of 3mol / L hydrochloric acid in 3 times, combine the water layers, wash with 50mL of dichloromethane, separate the organic layer, and wash the obtained aqueous solution with 20% sodium hydroxide The pH of the solution was adjusted to 2.5, a solid w...
Embodiment 3
[0022] Embodiment 3: the synthesis of compound 2
[0023] Under the protection of nitrogen, add 100mL of benzene, 40mmol of 7-ACA, 56mmol of hexamethyldisilazane, and 0.5mmol of pyridine in sequence in the reaction flask, stir and raise the temperature to reflux for 2h, cool the reaction solution to 0°C, dropwise add three Methyl iodosilane (TMSI) 48mmol, keep warm for 1h, add 44mmol of trifluoroethoxy phosphate, continue the reaction for 1h, add 40mmol of sodium hexamethyldisilazide, 80mmol of 15-crown-5, heat and reflux for 30min, cool to At room temperature, 40 mmol of acetaldehyde was added and reacted at room temperature for 6 h. After the reaction is complete, add 50mL of water to the reaction solution, stir, collect the organic phase, extract with 25mL of 3mol / L hydrochloric acid in 3 times, combine the water layers, wash with 50mL of dichloromethane, separate the organic layer, and wash the obtained aqueous solution with 20% sodium hydroxide The pH of the solution was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com