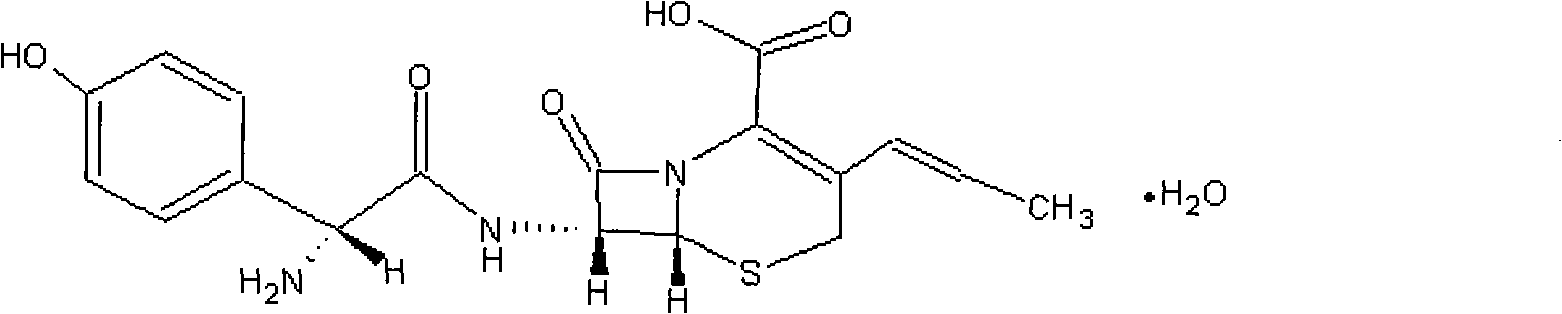
Cefprozil suspension pharmaceutical composition
A technology of cefprozil and composition, which is applied in the field of suspension pharmaceutical composition of cefprozil and xanthan gum, can solve the problems of poor redispersibility, long dispersion time, inconvenience for patients to take medicine, etc., and achieve good redispersibility, High product safety and good suspension effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1 adopts the xanthan gum of different viscosity to prepare the cefprozil dry suspension of different specifications
[0060] According to the method under the viscosity item of xanthan gum, the second part of the Chinese Pharmacopoeia 2010 edition, the viscosities of xanthan gum a, b, and c are about 1385mPa·s, 1840mPa·s and 2230mPa·s.
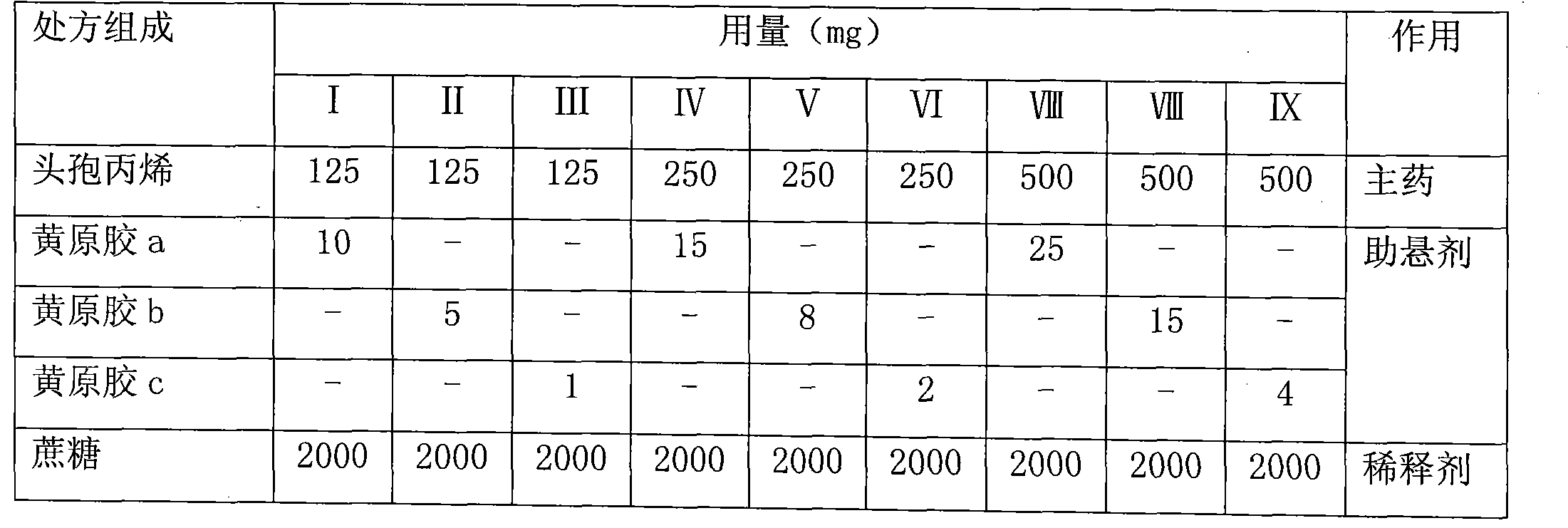
[0061] 1. The single-dose prescription contains 125mg, 250mg, and 500mg of cefprozil (based on anhydrous substance, crushed)
[0062] prescription:
[0063]
[0064] Process:
[0065] Weigh an appropriate amount of cefprozil and pulverize it to below 75 μm (equivalent to passing through a 200-mesh sieve), weigh 20 times the amount of raw and auxiliary materials according to each prescription and mix them evenly, and then divide them into two parts, one in powder state for later use, and the other The granules are made into granules by a dry granulator, and the granules below 80 mesh are collected for future use.
[0066...
Embodiment 2
[0093] Embodiment 2 adopts magnesium aluminum silicate of different viscosities and xanthan gum compatibility to prepare different specifications of cefprozil dry suspension
[0094] Xanthan gum with a viscosity of 1840mPa·s is used, and it is compatible with magnesium aluminum silicate a, b, and c with viscosities of 245mPa·s, 1202mPa·s, and 2317mPa·s respectively.
[0095] 1. The single-dose prescription contains 125mg, 250mg, 500mg of cefprozil (according to anhydrous substance, not pulverized)
[0096] prescription:
[0097]
[0098] Process:
[0099] Weigh 20 times the prescription amount of xanthan gum and magnesium aluminum silicate, first mix the two, and then mix with the remaining raw and auxiliary materials, and then make granules through a dry granulator, and collect the granules below 80 mesh for future use.
[0100] Detection indicators and methods:
[0101] With embodiment 1-1.
[0102] result:
[0103]
[0104] Note: 1- a few visible particles; 2- no...
Embodiment 3
[0117] Embodiment 3 On the basis of Example 1, different types of disintegrants are added to prepare single and multiple doses of cefprozil dry suspension
[0118] Xanthan gum with a viscosity of 1415mPa·s was used to carry out disintegrant screening test in combination with different disintegrants in a certain proportion.
[0119] Single dose of cefprozil dry suspension prescription containing main drug 125mg:
[0120]
[0121]
[0122] Prescription of multiple doses of cefprozil dry suspension containing 5g of the main drug:
[0123]
[0124] result:
[0125] The sedimentation volume ratios measured by the aforementioned method were all qualified, and no obvious sedimentation was seen after standing for 3 hours.
[0126]
[0127] The results showed that the preferred disintegrants were crospovidone, sodium starch glycolate and croscarmellose sodium.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com