Method for detecting zymoprotein residues in cefprozil prepared by enzymatic method
A technology for the preparation of cefprozil and enzymatic method, which is applied in the preparation of test samples and the measurement of color/spectral characteristics, etc., can solve the problems of patients' allergies, and achieve the effect of good reliability, high accuracy and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
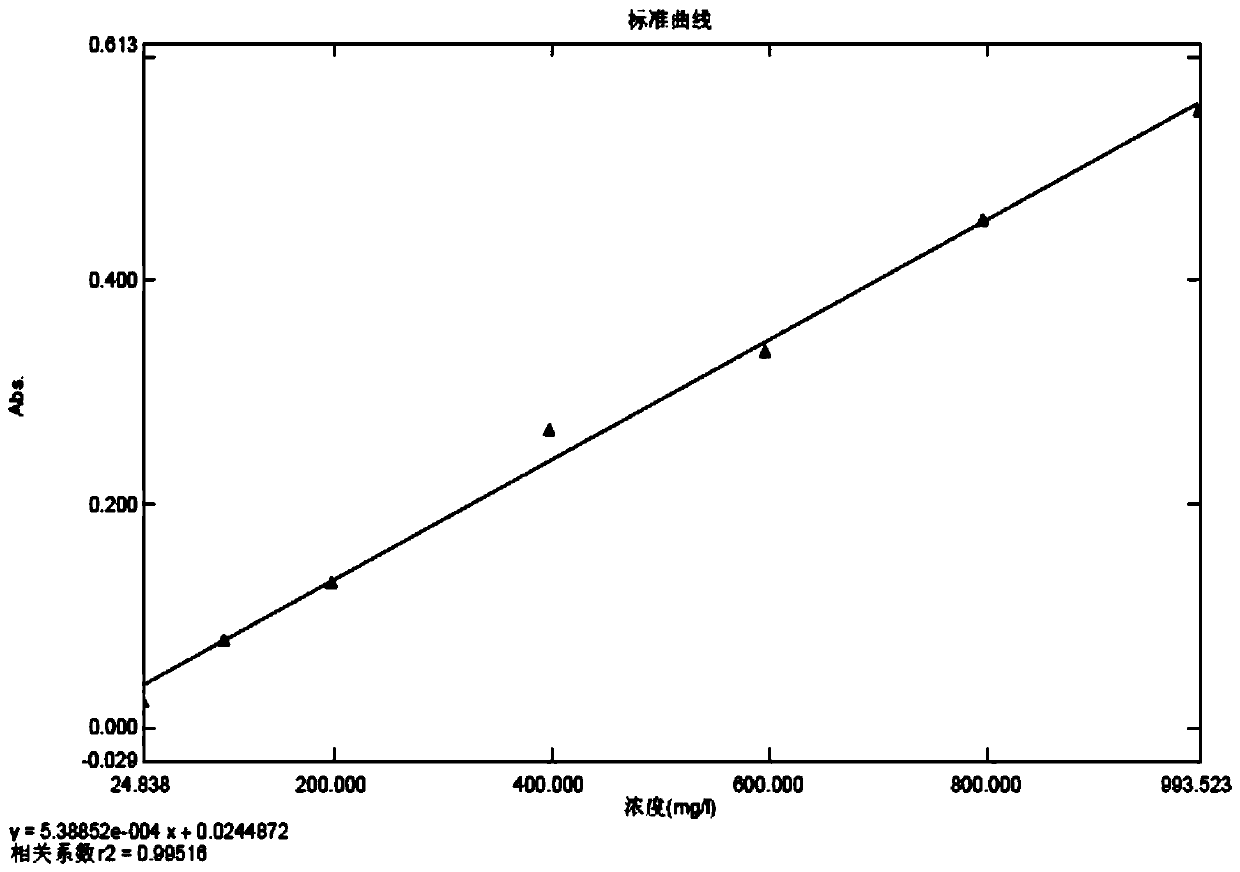
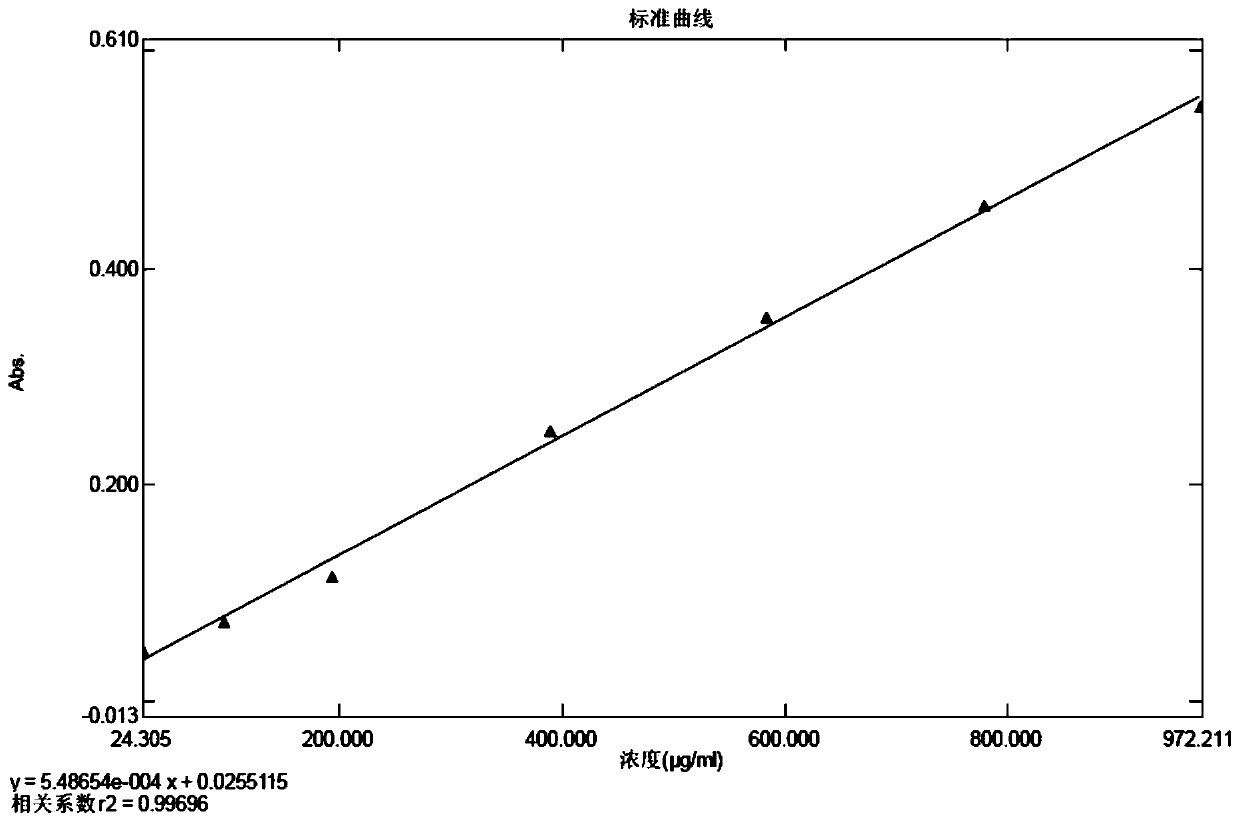
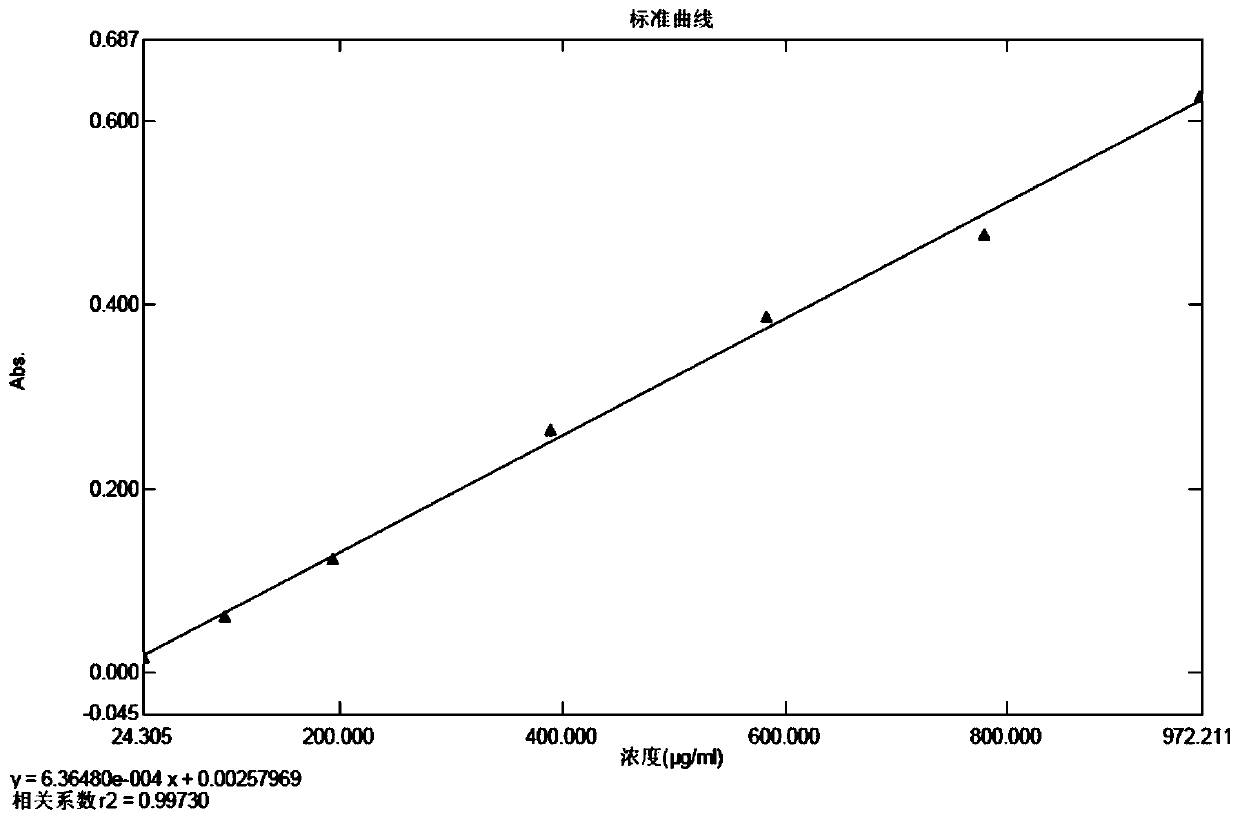
[0035] Preparation of the standard curve solution: take the standard protein stock solution and dilute it with sodium bicarbonate solutions of the same concentration and different volumes to obtain respective standard protein linear solutions; each take the same volume of the Coomassie Brilliant Blue G-250 dye solution, Add the above-mentioned standard protein linear solutions respectively, and mix well; after standing for reaction, the standard curve solution is obtained;
[0036] Preparation of the blank solution: Take the same volume of Coomassie Brilliant Blue G-250 staining solution as that used in the preparation of the standard curve solution, add the same concentration of sodium bicarbonate solution, mix well, leave it to react, and use it as a blank solution;
[0037] Preparation of the testing solution for the testing product: take an appropriate amount of cefprozil for the testing product, add the same concentration of sodium bicarbonate solution to dissolve and dilu...
Embodiment approach
[0040] As an embodiment, the preparation of Coomassie Brilliant Blue G-250 dye solution: take Coomassie Brilliant Blue G-250 and dissolve it in ethanol, then add an appropriate amount of phosphoric acid, and finally use purified water to make up the volume; preparation of standard protein stock solution: Weigh an appropriate amount of bovine serum albumin, dissolve and dilute it with purified water to obtain a standard protein stock solution.
[0041] As an embodiment, in the preparation process of the Coomassie Brilliant Blue G-250 dye solution, the ratio of the weight of Coomassie Brilliant Blue G-250, the volume of ethanol, the volume of phosphoric acid and the constant volume is 5g: (2~3)L : (5~6)L: 50L.
[0042] As an embodiment, the concentration of the standard protein stock solution is 0.1-5 mg / ml, and the concentration of the test solution is 5-100 mg / ml.
[0043] Further, the standing reaction time when the blank solution is prepared, the standing reaction time when...
Embodiment 1
[0056] The detection method of enzyme protein residue in the enzymatic preparation of cefprozil of the present embodiment comprises the following steps:
[0057] 1. Preparation of Assay Solution
[0058] (1) Preparation of Coomassie Brilliant Blue G-250 dye solution: Weigh 100 mg of Coomassie Brilliant Blue G-250, dissolve in 40-60 ml of ethanol, add 100-120 ml of phosphoric acid, and finally dilute to 1000 ml with purified water;
[0059] (2) Preparation of standard protein stock solution: 125 mg of bovine serum albumin was accurately weighed, dissolved in purified water to a volume of 50 ml, and used as a standard protein stock solution.
[0060] (3) Preparation of the standard curve solution: take the standard protein stock solution, dilute it with a sodium bicarbonate solution with a concentration of 10% g / ml as shown in Table 1, prepare 1 part of each concentration, and obtain 7 parts of the standard protein linear solution; Take 10ml of Coomassie Brilliant Blue G-250 dy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com