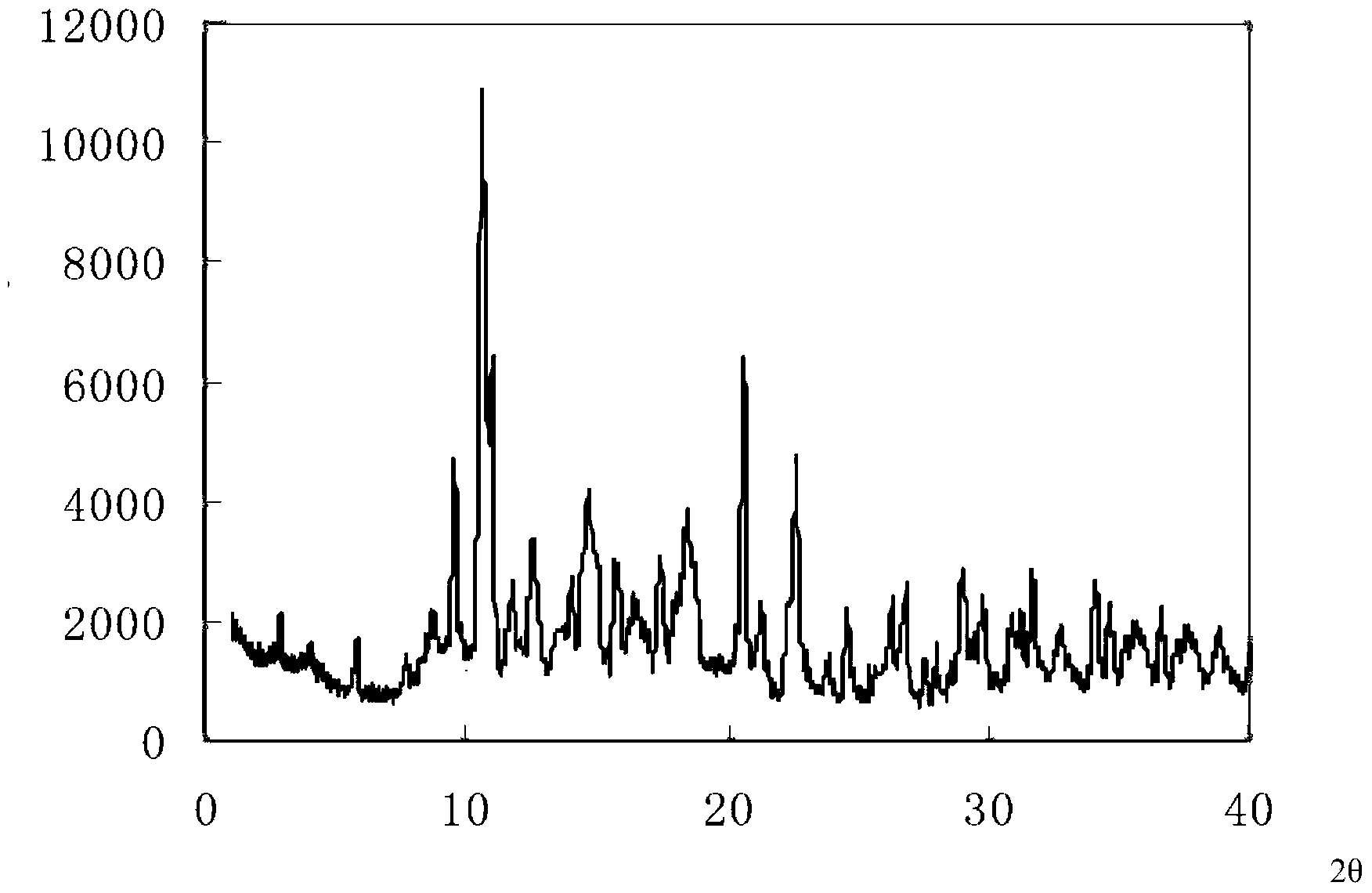
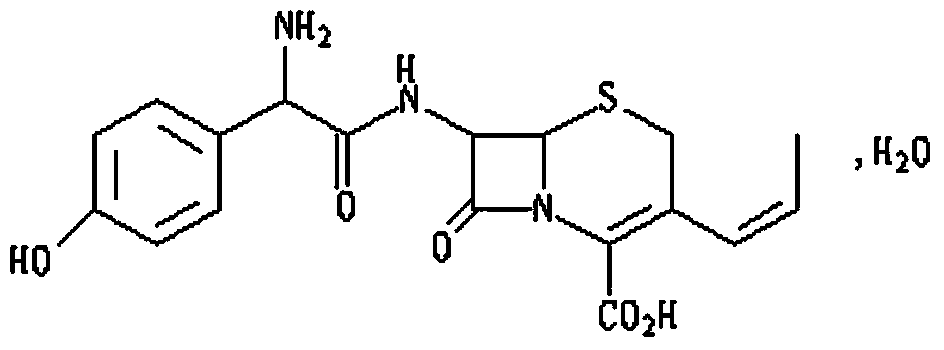
Cefprozil compound, and dispersible tablets, dry suspension and preparation method thereof
A technology of cefprozil and dry suspension, applied in the field of medicine, can solve the problems of decomposition and deterioration, affect the treatment effect of cefprozil, decrease and other problems, and achieve the effect of stable composition, suitability for clinical application and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: the preparation of cefprozil compound
[0051] 1. Prepare 5L of a saturated aqueous solution of crude cefprozil at 55°C;
[0052] 2. Prepare 30L of mixed organic solvent of ethanol, ether and ethyl acetate; the volume ratio of ethanol, ether and ethyl acetate in the mixed organic solvent is 4:1:1;
[0053] 3. Cool the organic solvent to 5°C. Under the sound field with a frequency of 25KHz and an output power of 40W, add the saturated aqueous solution of cefprozil crude product to the organic solvent at a constant speed while stirring. The adding speed is 3 liters / hour, and the stirring speed is 1200 rev / min, continue to stir and cool down after the addition, the stirring speed is 360 rev / min, the cooling rate is 1.5°C / hour; stop stirring after cooling down to 0°C, and let the crystal grow for 8 hours; filter after obtaining the crystal, and use Washed with water and ethanol, and dried in vacuum for 4 hours to obtain the cefprozil compound.
[0054] The p...
Embodiment 2
[0055] Embodiment 2: the preparation of cefprozil compound
[0056] 1. Prepare 5 L of saturated aqueous solution of cefprozil crude product at 60°C;
[0057] 2. Prepare 20L of mixed organic solvent of ethanol, ether and ethyl acetate; the volume ratio of ethanol, ether and ethyl acetate in the mixed organic solvent is 5:1:1;
[0058] 3. Cool the organic solvent to 0°C. Under a sound field with a frequency of 25KHz and an output power of 40W, add the saturated aqueous solution of cefprozil crude product to the organic solvent at a constant speed while stirring. The adding speed is 3 liters / hour, and the stirring speed is 600 rpm, continue to stir and cool down after the addition, the stirring speed is 60 rpm, and the cooling rate is 0.5°C / hour; stop stirring after cooling down to 0°C, and let the crystal grow for 6 hours; filter it after obtaining the crystal, and use Washed with water and ethanol, and dried under vacuum for 6 hours to obtain the cefprozil compound.
[0059] ...
Embodiment 3
[0060] Embodiment 3: Cefprozil dispersible tablet (specification 0.125g / tablet)
[0061] Its composition is:
[0062]
[0063] The preparation method is:
[0064] 1. Weigh the cefprozil compound and pharmaceutical adjuvant according to the formula; the cefprozil compound is prepared in Example 1 or 2;
[0065] 2. Sieving: Pass the cefprozil compound, sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose, and lactose through a 100-mesh sieve respectively;
[0066] 3. Mixing: Add cefprozil compound, sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose, and neotame into the mixer and mix for 20 minutes, then add sweet orange powdered essence, silicon dioxide, and spray-dried lactose to continue mixing 20 minutes, finally add magnesium stearate and mix for 10 minutes;
[0067] 4. Tablet, pack, and get ready.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Primary particle size | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Master granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com