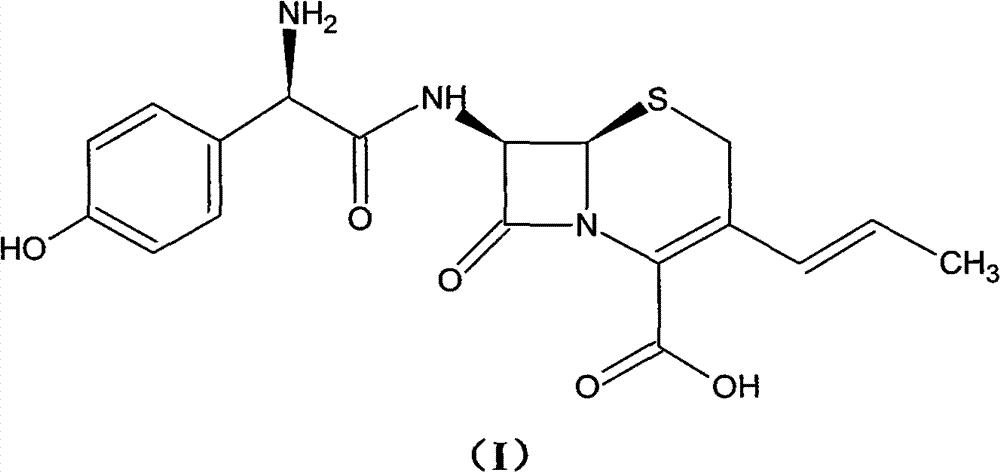
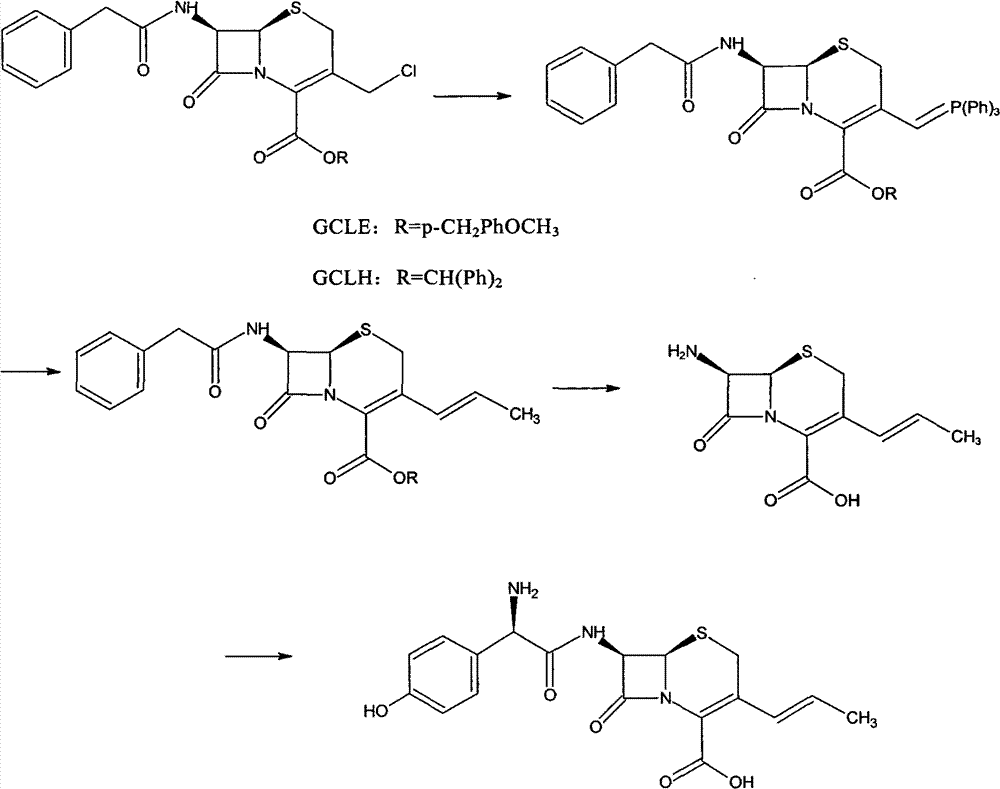
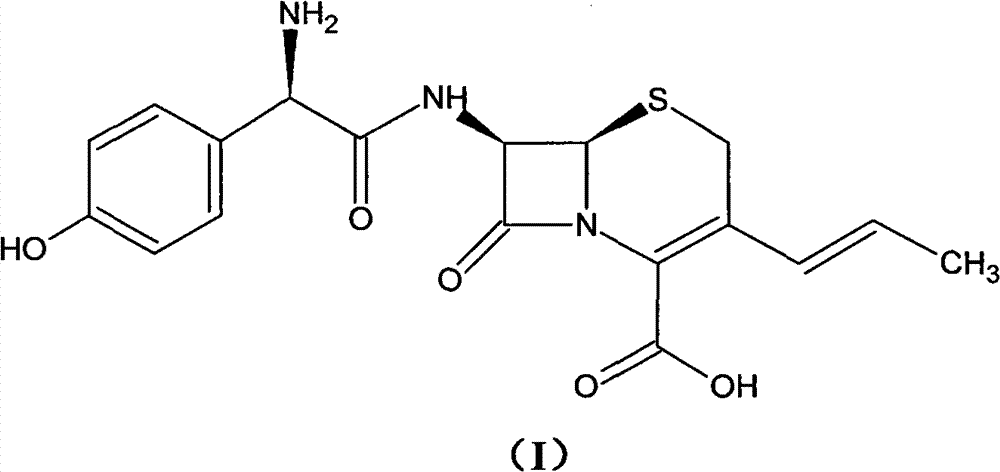
Preparation method of cefprozil
A technology of cefprozil and hydrate, which is applied in the field of synthesis of the second-generation cephalosporin antibiotic cefprozil, can solve the problems of low conversion rate, low product purity, and high content of trans isomers, and achieve low product loss , simple purification process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (1) Under nitrogen protection, GCLE50g, NaI15g, Ph 3 P30g was added into chloroform, and the reaction was stirred at 10°C. The end point of the reaction is monitored by the liquid phase. After the reaction is completed, the organic phase is obtained by washing with water and standing for separation. The organic phase was cooled to -5°C, and sodium hydroxide was added to react for 100 min, and the end point of the reaction was monitored by TCL. After the reaction was completed, the organic phase was obtained by standing and layering. The temperature of the organic phase was lowered to -10° C., and isopropanol and acetaldehyde were added to react for 18 h. After the reaction was completed, the organic phase was obtained by standing and separating. After the organic phase was washed with water, it was concentrated under reduced pressure until crystallization appeared, and 30 ml of isopropanol was added to stir, and the temperature was lowered to 0° C., filtered, and drie...
Embodiment 2
[0057] (1) Under nitrogen protection, GCLE50g, NaI16g, Ph 3 P30g was added into dichloromethane, and the reaction was stirred at 15°C. The end point of the reaction is monitored by the liquid phase. After the reaction is completed, the organic phase is obtained by washing with water and standing for separation. The organic phase was cooled to 0°C, and sodium hydroxide was added to react for 80 min, and the end point of the reaction was monitored by TCL. After the reaction was completed, the mixture was left to stand and separated to obtain an organic phase. The temperature of the organic phase was lowered to -15°C, and isopropanol and acetaldehyde were added to react for 16 hours. After the reaction was completed, 200 ml of 20% sodium thiosulfate solution was added, stirred, and left to stand for stratification to obtain an organic phase. After the organic phase was washed with water, it was concentrated under reduced pressure until crystallization appeared, and 30 ml of iso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com