Method for enzymatic synthesis of cefprozil in recyclable aqueous two-phase system by using immobilized penicillin acylase
A technology of penicillin acylase catalysis and penicillin acylase, which is applied in the direction of fermentation, can solve the problems of restricting large-scale production, inability to recycle, difficult to recycle, etc., and achieve increased yield, reduced by-products, and synthesis/hydrolysis ratio Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] at P NB / P ADB Enzyme-catalyzed synthesis of cefprozil in a reusable two-phase aqueous system:
[0037]To P NB / P ADB A mixture with a total volume of 10 ml was formed in the two aqueous solution. Two polymers P NB with P ADB The concentration in the system is 2.5% (w / w). Then, add 3g (780u) of penicillin G acylase, add 60mmol / L ammonium sulfate to adjust the distribution coefficient of the product cefprozil in the two aqueous phases, adjust the pH of the system to 6.5, and shake the reaction mixture at 15°C After reacting for 80 hours, the yield of cefprozil as determined by liquid chromatography reached 78%, and the post-treatment was carried out by acid precipitation. The final yield was consistent with the yield determined by liquid chromatography, and the solid cefprozil product was obtained by filtration.
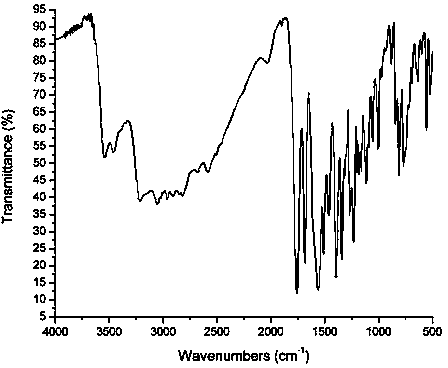
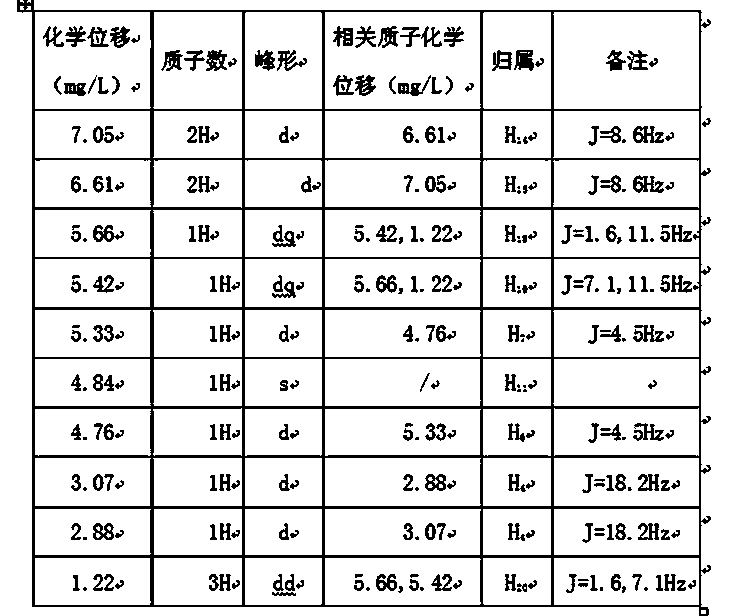
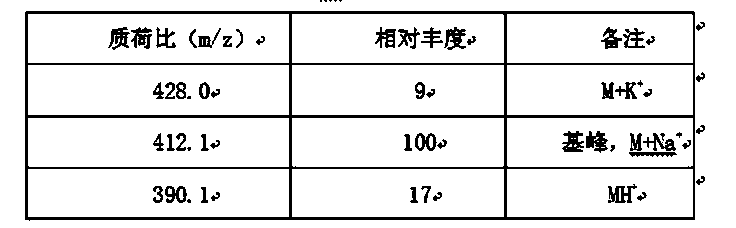
[0038] cefprozil 1 HNMR spectrum, mass spectrum and infrared spectrum (such as figure 1 shown). of which cefprozil 1 The HNMR data are shown in Tabl...
Embodiment 2
[0070] at P ADB / P ADBA Enzyme-catalyzed synthesis of cefprozil in a reusable two-phase aqueous system:
[0071] To P ADB / P ADBA A mixture with a total volume of 10 ml was formed in the two aqueous solution. Two polymers P ADB with P ADBA The concentration in the system is 2.5% (w / w). Then, add 3g (780u) of penicillin G acylase, add 60mmol / L ammonium sulfate to adjust the distribution coefficient of the product cefprozil in the two aqueous phases, adjust the pH of the system to 5.0, and shake the reaction mixture at 20°C After reacting for 80 hours, the yield of cefprozil determined by liquid chromatography reached 73%, and the post-treatment was carried out by acid precipitation method. The final yield was consistent with the yield determined by liquid chromatography, and the solid cefprozil product was obtained by filtration.
[0072] Cefprozil obtained through testing 1 HNMR data, mass spectral data and infrared spectrum data are the same as in Example 1.
Embodiment 3
[0074] at P ADB / Sodium citrate reusable enzyme-catalyzed synthesis of cefprozil in two-phase aqueous system:
[0075] To P ADB A mixture with a total volume of 10ml was formed in the aqueous sodium citrate two-phase solution, and the concentration of the two phase-forming substances in the system was 2.5% (w / w). Then, add 3g (780u) of penicillin G acylase, add 60mmol / L sodium dihydrogen phosphate to adjust the distribution coefficient of the product cefprozil in the two aqueous phases, adjust the pH of the system to 6.5, and place the reaction mixture at 20 The reaction was shaken at ℃ for 80 hours, and the yield of cefprozil as determined by liquid chromatography reached 75%. The acid precipitation method was used for post-treatment, and the final yield was consistent with the yield determined by liquid chromatography. The solid cefprozil product was obtained by filtration.
[0076] Cefprozil obtained through testing 1 HNMR data, mass spectral data and infrared spectrum d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com