Cefprozil mother liquor application technique
A technology of cefprozil and propylene mother liquor, which is applied in the field of medicine, can solve problems such as large solvent consumption and energy consumption, and product quality does not meet the requirements, and achieves significant economic and environmental benefits, convenient operation, and simple mother liquor application process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
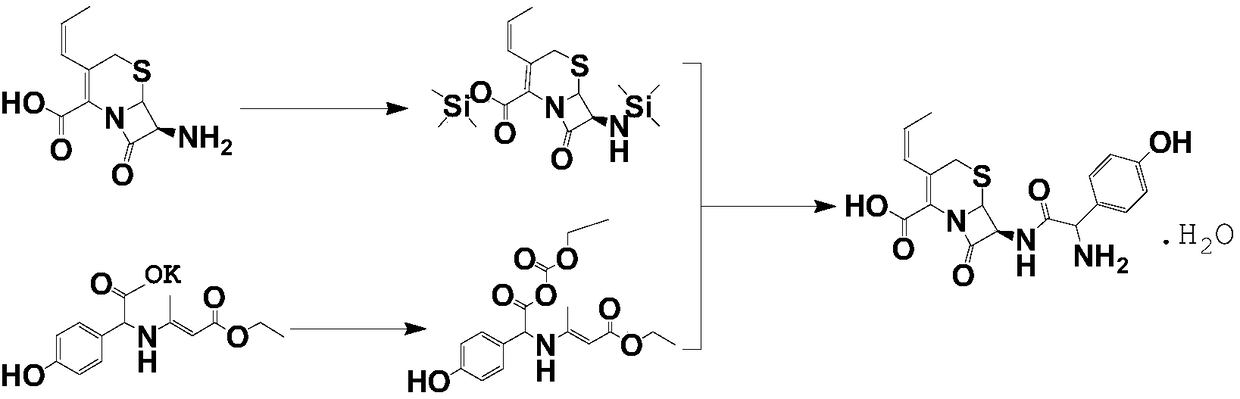
[0037] Take 28.4g of 7-APRA (cephalosporin core), add 200mL of dichloromethane, 15g of hexamethyldisilazane, 9.8g of trimethylchlorosilane and 1g of imidazole to three-necked flask A, and heat to reflux for 3h ;
[0038] Take 91.2g of p-hydroxyphenylglycine Deng potassium salt, add 200mL of dichloromethane and 150mL of DMF (dimethylformamide) in a three-necked flask B, drop the temperature to -50℃ and add ethyl chloroformate dropwise, control the temperature -33℃- React at 43℃ for 3h;
[0039] After the reaction, the active ester in the three-necked flask B was cooled to below -50°C, and the medium protective group mixture in the three-necked flask A was added dropwise. The temperature was controlled at -50°C and reacted for 2 hours to obtain cefprozil. Add 110mL crude cefprozil refined mother liquor and 9mL saturated FeCl to the reaction solution 3 The solution, add hydrochloric acid to adjust the pH value to 1.0, raise the temperature to 5℃-8℃, stir for 30min under temperature c...
Embodiment 2
[0042] Take 28.4g of 7-APRA, add 200mL of dichloromethane, 15.5g of hexamethyldisilazane, 9.8g of trimethylchlorosilane and 1g of imidazole to the three-neck flask A, and heat to reflux for 3h;
[0043] Take 91.2g of p-hydroxyphenylglycine Deng potassium salt, add 200mL of dichloromethane and 150mL of DMF to a three-necked flask B, drop the temperature to -50℃, add ethyl chloroformate, and control the temperature to -33℃-43℃ for 3h;
[0044] After the reaction, the active ester in the three-necked flask B was cooled to below -50°C, the protective group mixture in the three-necked flask A was added dropwise, and the temperature was controlled at -50°C for 2 hours to obtain cefprozil. The reaction to produce cefprozil Add 100mL crude cefprozil refined mother liquor and 11mL saturated FeCl 3 The solution, add hydrochloric acid to adjust the pH value to 0.8, raise the temperature to 8°C, stir for 30min, stand still, separate the liquids, cool the water phase to below -5°C, control the t...
Embodiment 3
[0047] Take 28.4g of 7-APRA, add 200mL of dichloromethane, 15.3g of hexamethyldisilazane, 9.8g of trimethylchlorosilane and 1g of imidazole to the three-necked flask A, and heat to reflux for 3h;
[0048] Take 91.2g of p-hydroxyphenylglycine Deng potassium salt, add 200mL of dichloromethane and 150mL of DMF to a three-necked flask B, drop the temperature to -50℃, add ethyl chloroformate, and control the temperature to -33℃-43℃ for 3h;
[0049] After the reaction, the active ester in the three-neck flask B was cooled to below -50°C, the protective group mixture in A was added dropwise, and the temperature was controlled at -50°C for 2 hours to prepare cefprozil. The cefprozil prepared reaction solution was added 120mL cef ℃ propylene crude refined mother liquor and 10mL saturated FeCl 3 The solution, add hydrochloric acid to adjust the pH value to 0.9, raise the temperature to 6, stir for 30min, stand still, separate, cool the water phase to below -5℃, add dimethylformamide and aceto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com