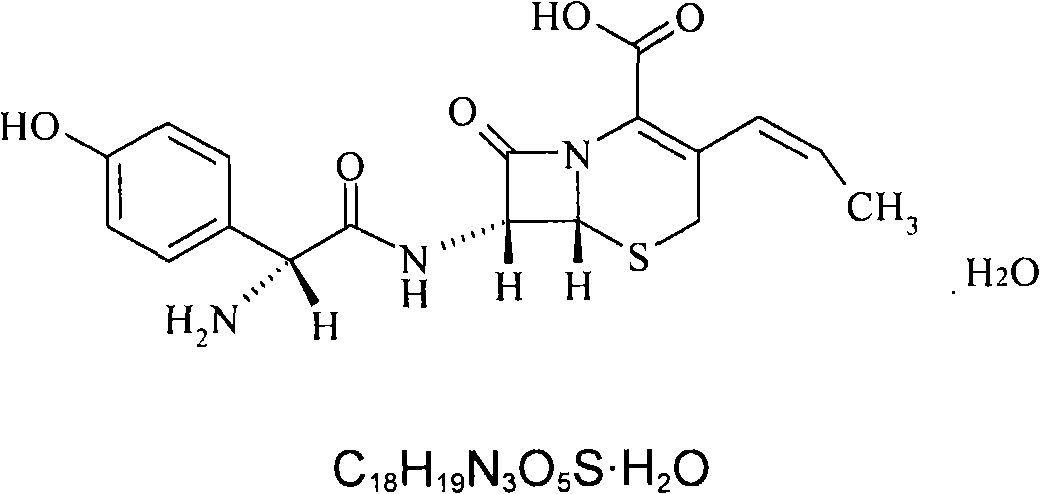
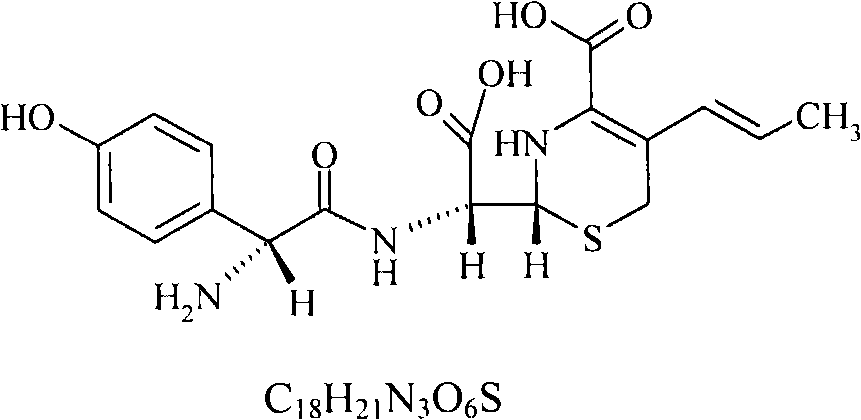
Cefprozil medicinal composition
A technology of cefprozil and composition, which is applied in the field of pharmaceutical compositions containing cefprozil and cellulose derivatives, and can solve the problems of drug inactivation and antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Mix 2000g of cefprozil, 240g of microcrystalline cellulose pH302 and 40g of hydroxypropyl cellulose LH11 in a three-dimensional mixer for 50-70 minutes until the cefprozil is fully dispersed and adhered to the cellulose derivatives; In the press, the pressure is adjusted to 0.5-5 MPa, the rotating speed is 200-300 rpm, and pressed into a hard sheet; the sheet can be directly pressed into a tablet.
[0041] According to requirements, the obtained flakes are crushed with a granulator or pulverizer, and passed through a 18-100 mesh sieve to obtain the powder of the cefprozil pharmaceutical composition of the present invention.
Embodiment 2~13
[0043] According to the method of Example 1, the cefprozil pharmaceutical compositions of Examples 2-13 were prepared using the composition shown in Table 1 below.
[0044] Table 1 (unit: g)
[0045]
[0046]
[0047]
Embodiment 14
[0049] formula:
[0050] Cefprozil 2000g
[0051] Cellulose derivatives: microcrystalline cellulose pH302 240g
[0052] Hypromellose LH11 40g
[0053] Thinner: 400g pregelatinized starch
[0054] Disintegrant: croscarmellose sodium 120g
[0055] Lubricant: Magnesium Stearate 20g
[0056] Talc powder 80g
[0057] Micronized silica gel 32g
[0058] Preparation method: Mix cefprozil, microcrystalline cellulose pH302 and hydroxypropyl cellulose LH11 in a three-dimensional mixer for 50 to 70 minutes until cefprozil is fully dispersed and adhered to cellulose derivatives; put the above mixture into dry In the press, the pressure is adjusted to 0.5-8 MPa, the speed is 200-300 rpm, and pressed into a hard sheet.
[0059] The resulting flakes are crushed with a granulator or pulverizer, mixed with pregelatinized starch, croscarmellose sodium, magnesium stearate, talc powder and micropowder silica gel in a three-dimensional mixer for 10 to 20 minutes, After mixing uniformly, eac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com