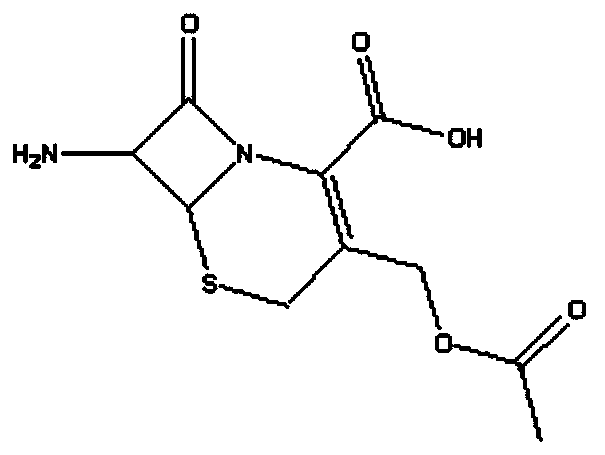
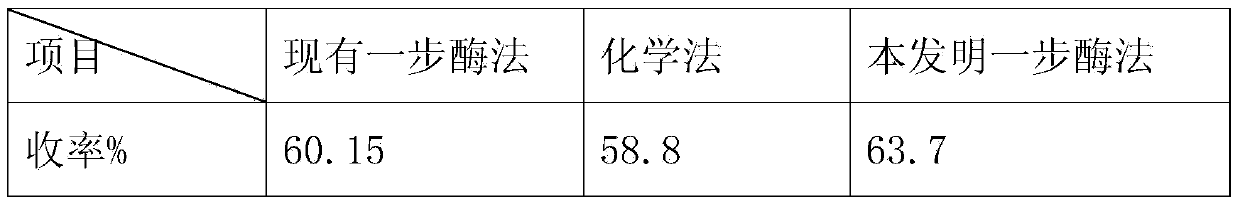
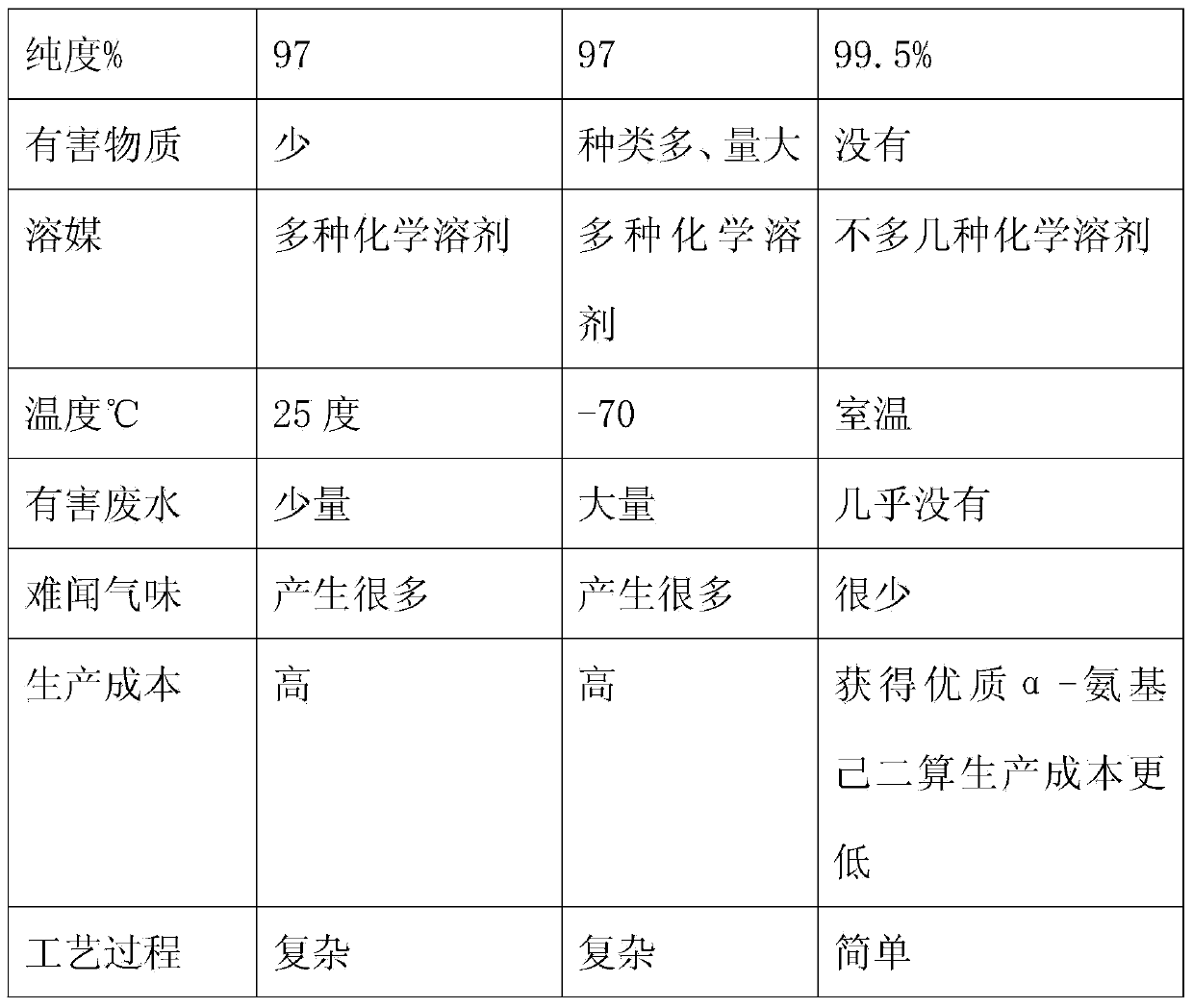
Method for preparing 7-ACA (aminocephalosporanic acid) and obtaining alpha-aminoadipic acid by one-step enzymatic reaction
A technology of aminoadipic acid and cephalosporin, applied in the direction of fermentation, can solve the problems of difficult control of the process, insufficient yield, purity and cost of the two-step enzymatic method, and achieve mild, safe and fast reaction conditions, and eliminate waste liquid. The effect of pollution, simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Take 10 liters of cephalosporin C (CPC) fermentation filtrate (750mM, equivalent to 311 grams of pure CPC), filter through a 1μm membrane, and adjust the pH to 10 with ammonia water.
Embodiment 2
[0058] Embodiment 2 reaction conditions control:
[0059] Add (NRB-103) enzyme 1800g to the reactor, wash twice with water, add CPC solution, under rapid stirring, control pH to 8-10 with ammonia water, react for 30 minutes, 7-ACA conversion rate is 98%.
Embodiment 3
[0060] The processing of embodiment 3 lysate (7-ACA liquid):
[0061] To the resulting 15 liters of 7-ACA solution was added 100 g of Na 2 S 2 o 4 , adjust the pH to 7 with ammonia water, add Tween 80, 10 ml, add 2000 ml of dichloromethane, stir for 10 minutes, separate phases, add 20 grams of activated carbon to the water phase, add 10 grams of EDTA, stir for 15 minutes, filter to remove carbon 7-ACA treatment solution was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com