Synthetic method of 7-MAC intermediate
A synthesis method, the technology of metoxycephalosporin, applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of 7-TMCA synthesis method not mentioned, low reactivity, insufficient reaction, etc., to reduce production costs, reduce costs, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
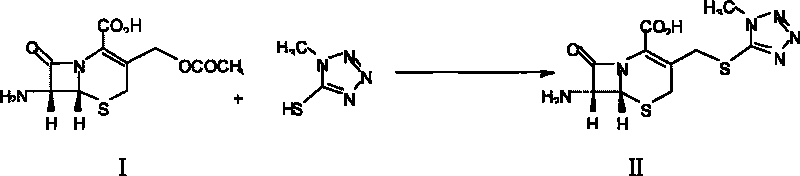
[0023] (1), Synthesis of 7-TMCA: In a 1000ml four-necked reaction flask, add 21.74 grams of 7-ACA, 13 grams of methylmercaptotetrazolium, and 200 grams of acetonitrile, stir evenly, cool to 0°C to 5°C, add concentrated Sulfuric acid 180g, react at 30°C for 3 hours, after the reaction, cool the reaction liquid to 0°C-5°C, add 750g of purified water dropwise, control the temperature during the dropwise addition to less than 20°C, add concentrated ammonia water dropwise to adjust the pH value to 4.0, and control the temperature When the temperature is lower than 20°C, stir for 30 minutes and grow crystals for 1 hour. Then filter, rinse twice with water and acetone respectively to obtain a wet product, vacuum-dry at below 40°C until the weight content of water is less than 1%, and the powder is collected to obtain about 22 grams of the product.
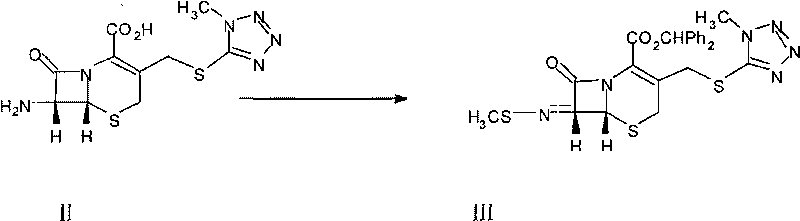
[0024] (2), the synthesis of formula III intermediate:
[0025] A. Preparation of methyl sulfide bromide: Add 16.4 grams of dimethyl di...
Embodiment 2
[0032] (1), the synthesis of 7-TMCA is the same as in Example 1.
[0033] (2), the synthesis of formula III intermediate:
[0034] The preparation of A, methyl thio bromide is the same as in Example 1.
[0035] B, the preparation of diphenyldiazomethane is the same as in Example 1.
[0036] C. Synthesis of the intermediate of formula III: 1000ml four-neck bottle with reflux condenser, put 22 grams of 7-TMCA, stir for 10 minutes with an appropriate amount of dichloromethane, add 40 grams of hexamethyldisilamine (HMDS) dropwise, and react for 4 hours , the rest of the operations were the same as in Example 1, and about 29.5 grams of the intermediate of formula III were obtained, the HPLC purity was ≥99%, and the moisture content was ≤0.8% (weight).
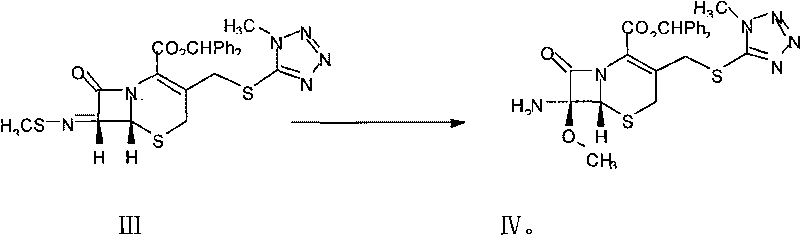
[0037] (3), the synthesis of 7-MAC is the same as in Example 1.
Embodiment 3
[0039] (1), the synthesis of 7-TMCA is the same as in Example 1.
[0040] (2), the synthesis of formula III intermediate:
[0041] The preparation of A, methyl thio bromide is the same as in Example 1.
[0042] B, the preparation of diphenyldiazomethane is the same as in Example 1.
[0043] C, the synthesis of formula III intermediate: in this step, use the mixed solution of trimethyl monochlorosilane (TMCS), diazabicyclo (DBU) and trimethyl iodosilane (TMIS) as carboxyl protecting agent, the mixing The volume ratio of each composition of liquid is 1: 1: 1, and all the other are identical with embodiment 1 this step.
[0044] (3), the synthesis of 7-MAC is the same as in Example 1.
[0045] The HPLC purity of the 7-MAC obtained in this embodiment is ≥98%, the light transmittance is ≥80%, and the moisture content is ≤0.5% (weight).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com