Preparation technology of cefotaxime
A preparation process, the technology of cefotaxime, applied in the field of preparation of pharmaceutical compounds, can solve the problems of long production cycle, poor crystal form, prolonging the production cycle, etc., to achieve the expansion of equipment input, shorten the production cycle, and reduce production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
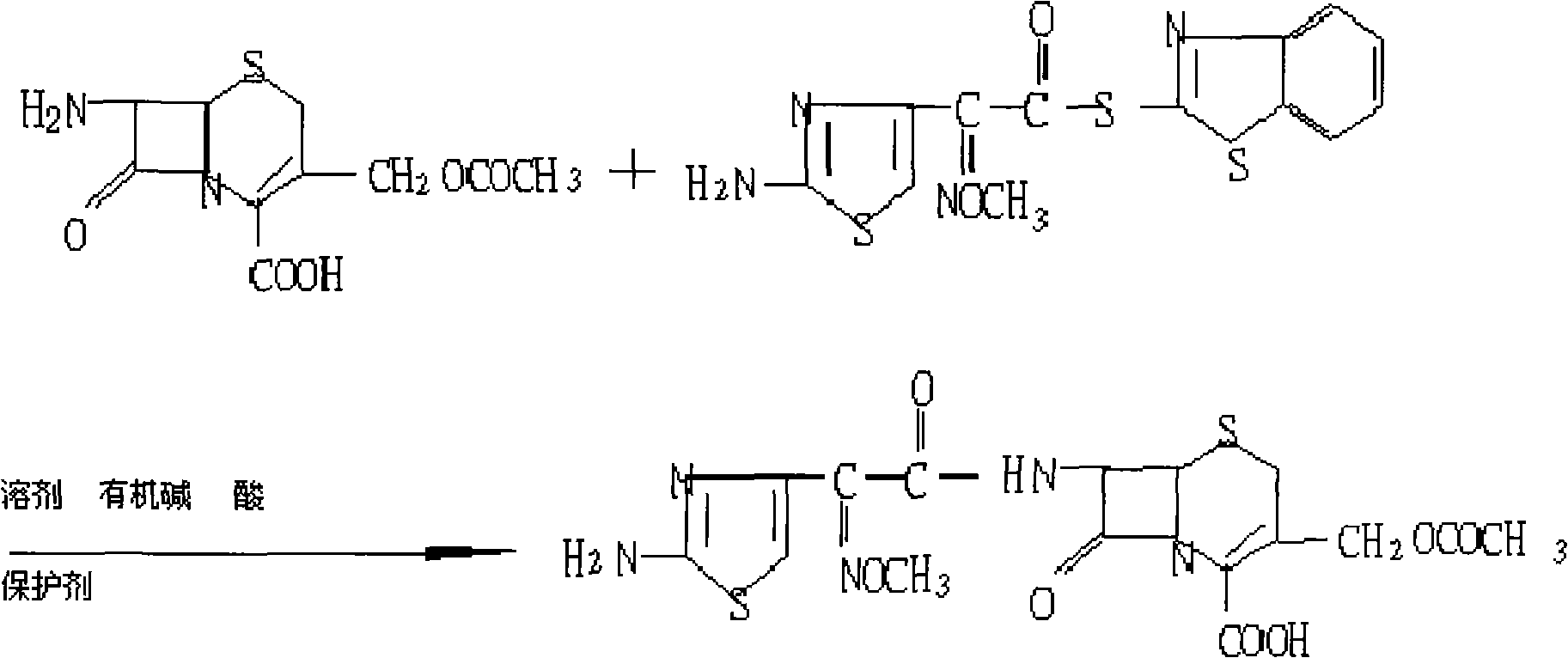
[0017] The preparation technology of cefotaxime of the present invention is as follows:
[0018] Synthesis reaction: Add 100ml of dichloromethane to a clean and dry three-necked bottle, cool down to 3 degrees, add 10ml of ethanol, control the temperature below 5 degrees, add 19ml of triethylamine, control the temperature at 8 degrees, add 20g of 7-ACA, 27g of AE-active Ester, react at a controlled temperature of about 15°C until the reaction is clear.
[0019] Acidification and crystallization: add hydrochloric acid to the above reaction solution, adjust the pH value to 2.22, cool down and grow crystals for 60 minutes, filter with suction below 10°C, stir and wash the filter cake once with ethanol, and rinse once. After being pumped dry, it was air-dried to obtain cefotaxime crystalline finished product. The yield of the product is 98.8%, the color is 3Y(-), and other items are in line with the internal control requirements of the enterprise.
Embodiment 2
[0021] The preparation technology of cefotaxime of the present invention is as follows:
[0022] Synthesis reaction: Add 100ml of dichloromethane to a clean and dry three-necked bottle, cool down to 5 degrees, add 17ml of isopropanol, control the temperature below 5 degrees, add 12ml of pyridine, control the temperature at 10 degrees, add 20g of 7-ACA, 27.5g of AE- Active ester, control the temperature to react at about 17°C until the reaction is clear.
[0023] Acidification and crystallization: add a mixture of hydrochloric acid and ethanol to the above reaction solution, adjust the pH value to 2.0, lower the temperature, grow the crystal for 60 minutes, filter with suction below 10°C, and wash the filter cake once with acetone and rinse once. After being pumped dry, it was air-dried to obtain cefotaxime crystalline finished product. The product yield is 99.8%, and the color 3Y(-) and other items all meet the internal control requirements of the enterprise.
Embodiment 3
[0025] The preparation technology of cefotaxime of the present invention is as follows:
[0026] Synthesis reaction: Add 100ml of chloroform to a clean and dry three-necked bottle, cool down to 8 degrees, add 20ml of isopropanol, control the temperature below 5 degrees, add 19ml of triethylamine, control the temperature at 8 degrees, add 20g of 7-ACA, 27.5 gAE-active ester, control the temperature at about 14°C until the reaction is clear.
[0027] Acidification and crystallization: add hydrochloric acid to the above reaction solution, adjust the pH value to 3.0, lower the temperature, grow the crystal for 60 minutes, filter with suction below 10°C, stir and wash the filter cake once with ethanol, and rinse once. After being pumped dry, it was air-dried to obtain cefotaxime crystalline finished product. The product yield is 100.1%, the color is 3Y, and other items are in line with the internal control requirements of the enterprise.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com