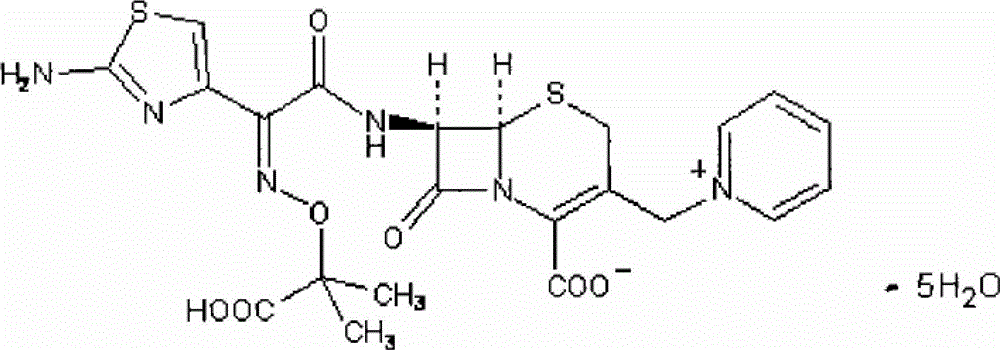
Synthesis of antibiotic ceftazidime, ceftazidime for injection and preparation method of ceftazidime
A technique for ceftazidime and its synthetic method, which is applied in the field of antibiotic drug preparation, can solve the problems of ceftazidime, such as difficult and accurate quantification, large particle size, and uneven mixing, and is suitable for large-scale industrial production, with low impurity content and good fluidity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
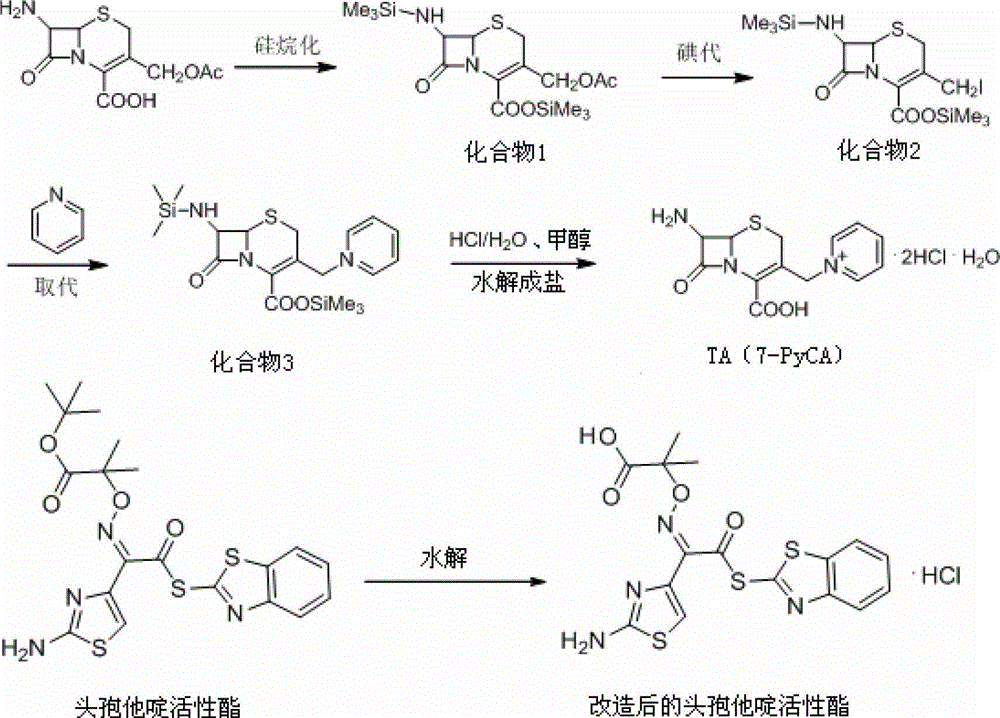
[0042] Synthesis of TA (7-PyCA)
[0043] Into the flask, add 55g of 7-ACA, 45g of hexamethyldisilane, and 1ml of activator trimethylchlorosilane, control the temperature at 40°C~50°C, and keep the reaction for 4 hours to obtain compound 1.
[0044] To compound 1, 65 g of iodotrimethylsilane was slowly added dropwise over 50 to 60 minutes, the addition temperature was controlled at 12°C to 15°C, and the reaction was kept for 2 hours to obtain compound 2.
[0045]In the flask, 35 g of pyridine was slowly added dropwise over 40 to 45 minutes, the temperature was controlled at 3°C to 5°C, and the reaction was kept for 1 hour to obtain compound 3.
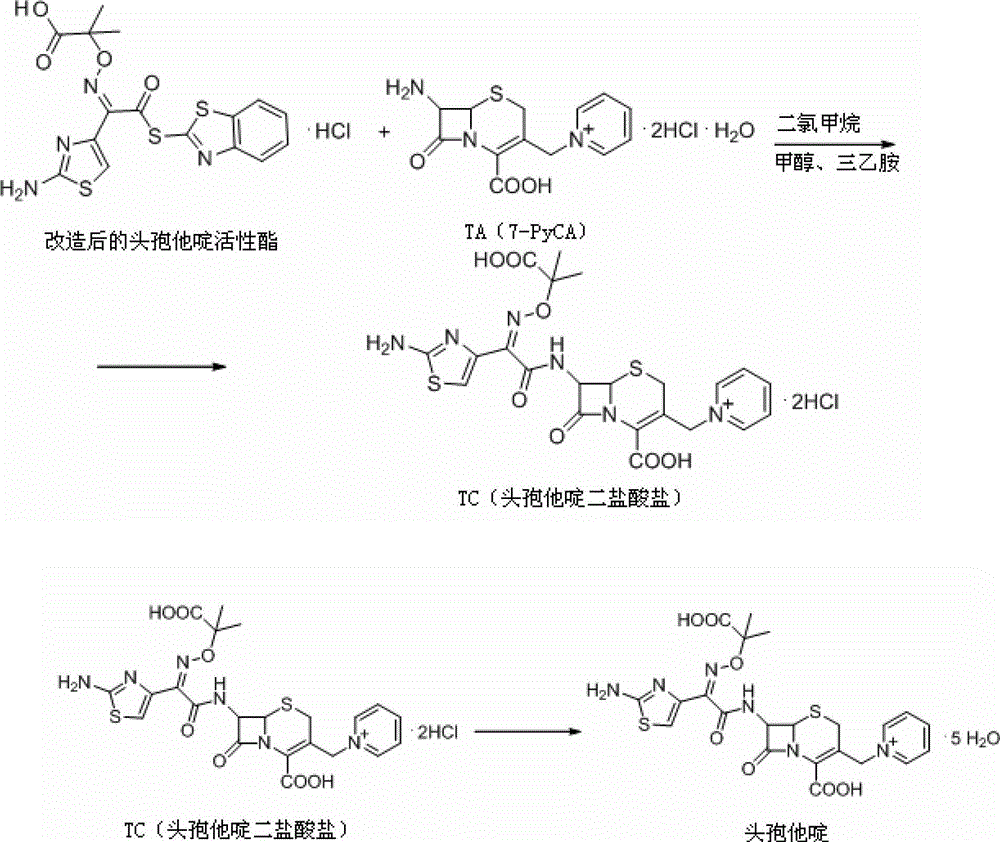
[0046] To compound 3, slowly add 45g of methanol dropwise over 60 minutes, control the temperature at 10°C~15°C, add a mixed solution consisting of 100g of hydrochloric acid and 100g of water, stir for 30 minutes, carry out crystal growth for 30 minutes, and continue stirring for 2 hours to obtain compound TA (7-PyCA), the mass yield...
Embodiment 2
[0054] Synthesis of TA (7-PyCA)
[0055] Into the flask, add 55g of 7-ACA, 45g of hexamethyldisilane, and 1ml of trimethylchlorosilane as an active agent, control the temperature at 20°C to 30°C, and keep the reaction for 4 hours to obtain compound 1.
[0056] To compound 1, 65 g of iodotrimethylsilane was slowly added dropwise over 50 to 60 minutes, the addition temperature was controlled at 10°C to 11°C, and the reaction was kept for 2 hours to obtain compound 2.
[0057] In the flask, 35 g of pyridine was slowly added dropwise over 40 to 45 minutes, the temperature was controlled at 0°C to 3°C, and the reaction was kept for 1 hour to obtain compound 3.
[0058] To compound 3, slowly add 45g of methanol dropwise over 60 minutes, control the temperature at 0°C~5°C, add a mixed solution consisting of 100g of hydrochloric acid and 100g of water, stir for 30 minutes, grow crystals for 30 minutes, and continue stirring for 2 hours to obtain compound TA (7-PyCA), the mass yield w...
Embodiment 3
[0066] Synthesis of TA (7-PyCA)
[0067] Into the flask, add 55g of 7-ACA, 45g of hexamethyldisilane, and 1ml of trimethylchlorosilane as an active agent, control the temperature at 60°C to 80°C, and keep the reaction for 4 hours to obtain compound 1.
[0068] To compound 1, slowly add 65 g of iodotrimethylsilane dropwise over 50 to 60 minutes, control the feeding temperature at 15°C to 20°C, and keep the reaction for 2 hours to obtain compound 2.
[0069] In the flask, 35 g of pyridine was slowly added dropwise over 40 to 45 minutes, the temperature was controlled at 8°C to 10°C, and the reaction was kept for 1 hour to obtain compound 3.
[0070] To compound 3, slowly add methanol 45g dropwise over 60 minutes, control the temperature at 15°C~20°C, add a mixed solution consisting of 100g hydrochloric acid and 100g water, stir for 30 minutes, carry out crystal growth for 30 minutes, and continue stirring for 2 hours to obtain compound TA (7-PyCA), the mass yield was 1.29%.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com