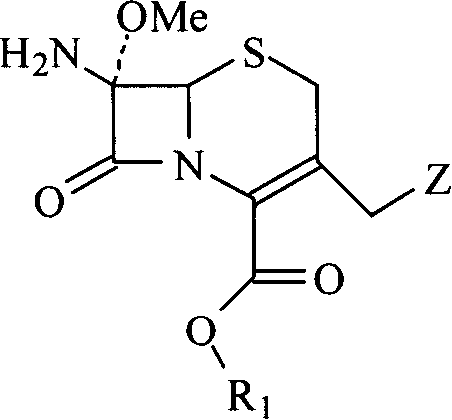
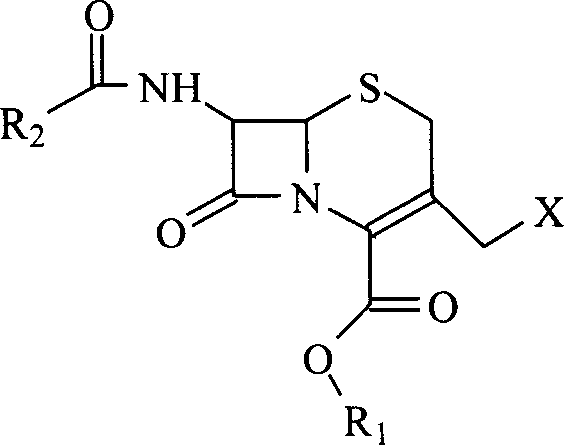
Methoxy cephalosporin intermediate
A methoxy head, cephalosporin technology, applied in bulk chemical production, organic chemistry and other directions, can solve the problems of low reactivity, complex reaction, long deprotection route and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 7-Phenylacetamido-3-vinyl-4-cephemic acid p-methoxybenzyl ester
[0024]Add 10 g of p-methoxybenzyl 7-phenylacetamido-3-chloromethyl-4-cephemate, 3 g of NaI, 5.93 g of triphenylphosphine, and 60 mL of acetone into a five-neck flask, and stir at 20°C for 1 h. Then 60 mL of dichloromethane and 17 mL of 37% formaldehyde aqueous solution were added, and the temperature was lowered to 15°C. Keeping this temperature, 20 mL of 1 mol / L sodium hydroxide solution was added dropwise. Continue stirring for 30 min after dropping, pour the mixture into 60 mL of 0.1 mol / L hydrochloric acid, stir for 10 min, and separate the organic phase. The aqueous phase was extracted with 60 mL of dichloromethane. The organic phases were combined and washed with 100 mL of water. Concentrate the organic phase under reduced pressure at 25°C, evaporate part of the solvent, add 150 mL of methanol preheated to 30°C, add seed crystals, and crystallize after 30 minutes. Concentration was continued, pa...
Embodiment 2
[0026] 7-Phenoxyacetylamino-3-pyridylmethyl-4-cephalosporanic acid benzhydryl ester
[0027] Dissolve 10 g of 7-phenoxyacetamido-3-iodomethyl-4-cephalosporanic acid diphenylmethyl ester in 80 mL of dichloromethane, adjust the temperature to 8-10 °C, add 4 mL of pyridine dropwise, complete the addition in 10 minutes, and raise the temperature to React at 20-25°C for 2 hours, after the reaction is complete, lower the temperature to 0-5°C, add 100mL of isopropyl ether, stir for 1h, filter with suction, wash the filter cake with 20mL of isopropyl ether, drain it, and dry it in vacuum at room temperature for 6h to obtain a light yellow powder 7-Phenoxyacetylamino-3-pyridylmethyl-4-cephalosporanic acid diphenylmethyl ester, the yield is 90%.
Embodiment 3
[0029] 7-Naphthylacetamido-7α-methoxy-3-(1-methyl-1,3,4,5-tetrazolium)thiomethyl-4-cephenoic acid p-nitrobenzyl ester
[0030] Add 50mL of treated anhydrous methanol to a 250mL three-neck flask, protect it with nitrogen, cool it to below -80 degrees with liquid nitrogen, add 1.0g lithium methoxide, and add 5.9g 7-naphthylacetamido-3-(1-methyl -1,3,4,5-tetrazolium)thiomethyl-4-cephemic acid p-nitrobenzyl ester was dissolved in 50mL of anhydrous tetrahydrofuran, and added dropwise to the reaction system at a relatively fast speed, while ensuring that the system temperature was at -80 below the degree. After 5 minutes, 1.5 mL of tert-butyl hypochlorite was added. Stir at -80°C for 30 minutes. Trimethyl phosphite and glacial acetic acid were then added. The temperature was raised to room temperature, the solvent was evaporated, dissolved in dichloromethane, washed successively with saturated sodium chloride, sodium bicarbonate and saturated sodium chloride. After drying over a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com