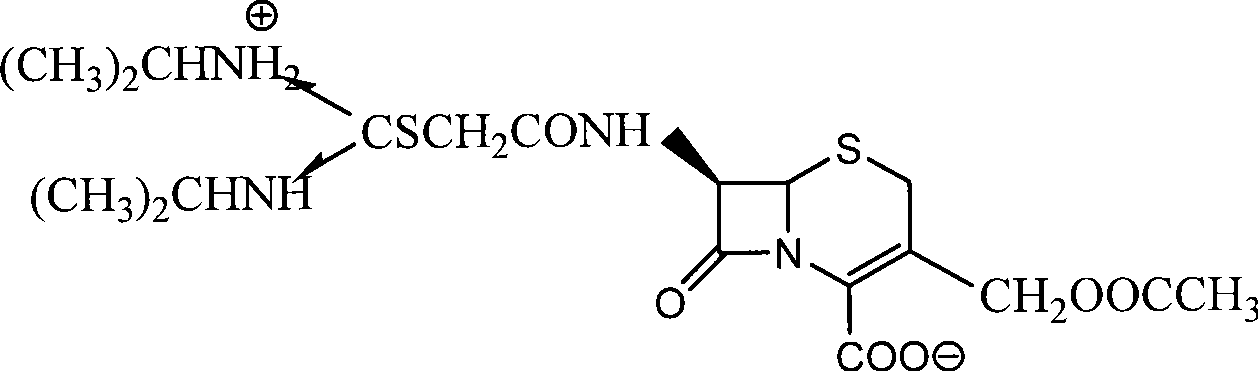
Process for preparing cefathiamidine
A technology of cefathiamidine and diisopropylthiourea, which is applied in the fields of organic chemistry and antibacterial drugs, can solve the problems that chlorine is not as active as bromine, the reaction difficulty is increased, and the reaction time is long, so as to facilitate storage and transportation , more stable quality and shorter steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
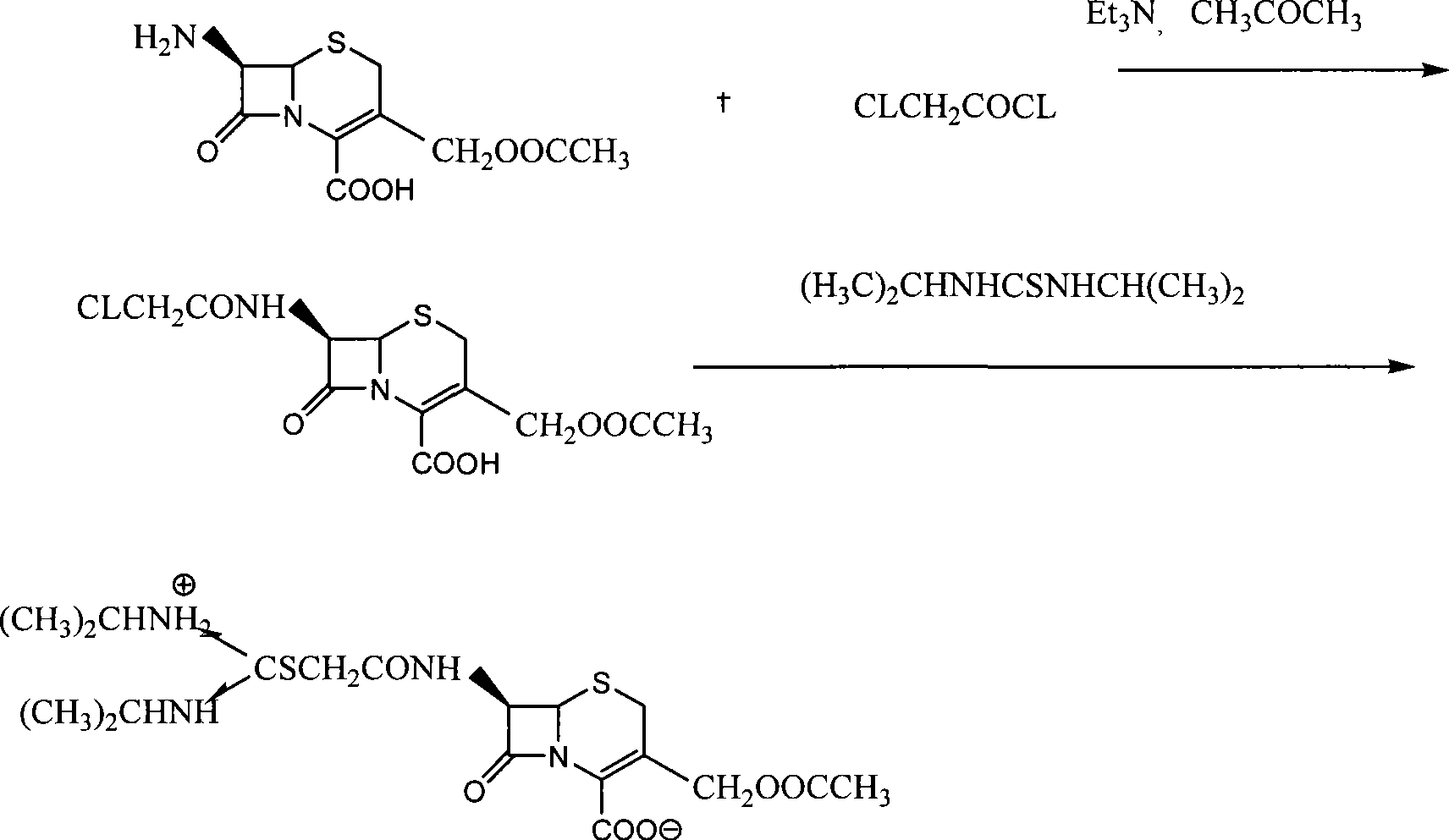
[0024] The preparation of embodiment 1 chloroacetyl 7-ACA
[0025] Add 600 milliliters of acetone and 100 milliliters of water into the reaction vessel, add 100 grams of 7-ACA and 40 grams of triethylamine at 0-5 ° C, after dissolving, add 50 milliliters of chloroacetyl chloride dropwise, and then add 70 grams of triethylamine dropwise , heat up to 15-25°C, and react for 1 hour; then, add 500 ml of water to the reaction solution, adjust the pH to 4-4.5 with 6N hydrochloric acid, control the internal temperature at about 20°C, and distill acetone under reduced pressure; Continue to adjust the pH to 1 with 6N hydrochloric acid at 5°C, filter, and wash the product twice with water. Vacuum-dried to obtain white solid chloroacetyl 7-ACA with a yield of 84-87%, and the content of chloroacetyl 7-ACA measured by HPLC was greater than 95%.
Embodiment 2
[0026] The preparation of embodiment 2 chloroacetyl 7-ACA
[0027] Add 300ml of ethanol and 900ml of water into the reaction vessel, add 100g of 7-ACA at 1-5°C, stir, add 150g of sodium bicarbonate to dissolve 7-ACA, then slowly add 70ml of chloroacetyl chloride dropwise , heated to 25-30°C, and reacted for 0.5 hours; then, 6N hydrochloric acid was added dropwise to the reaction liquid to adjust the pH of the solution to 1, stirred for 1 hour, filtered, and the product was washed twice with water. Vacuum-dried to obtain white solid chloroacetyl 7-ACA, the yield is 76-80%, and the content measured by HPLC method is greater than 92%.
Embodiment 3
[0028] Example 3 Preparation of Cefathiamidine
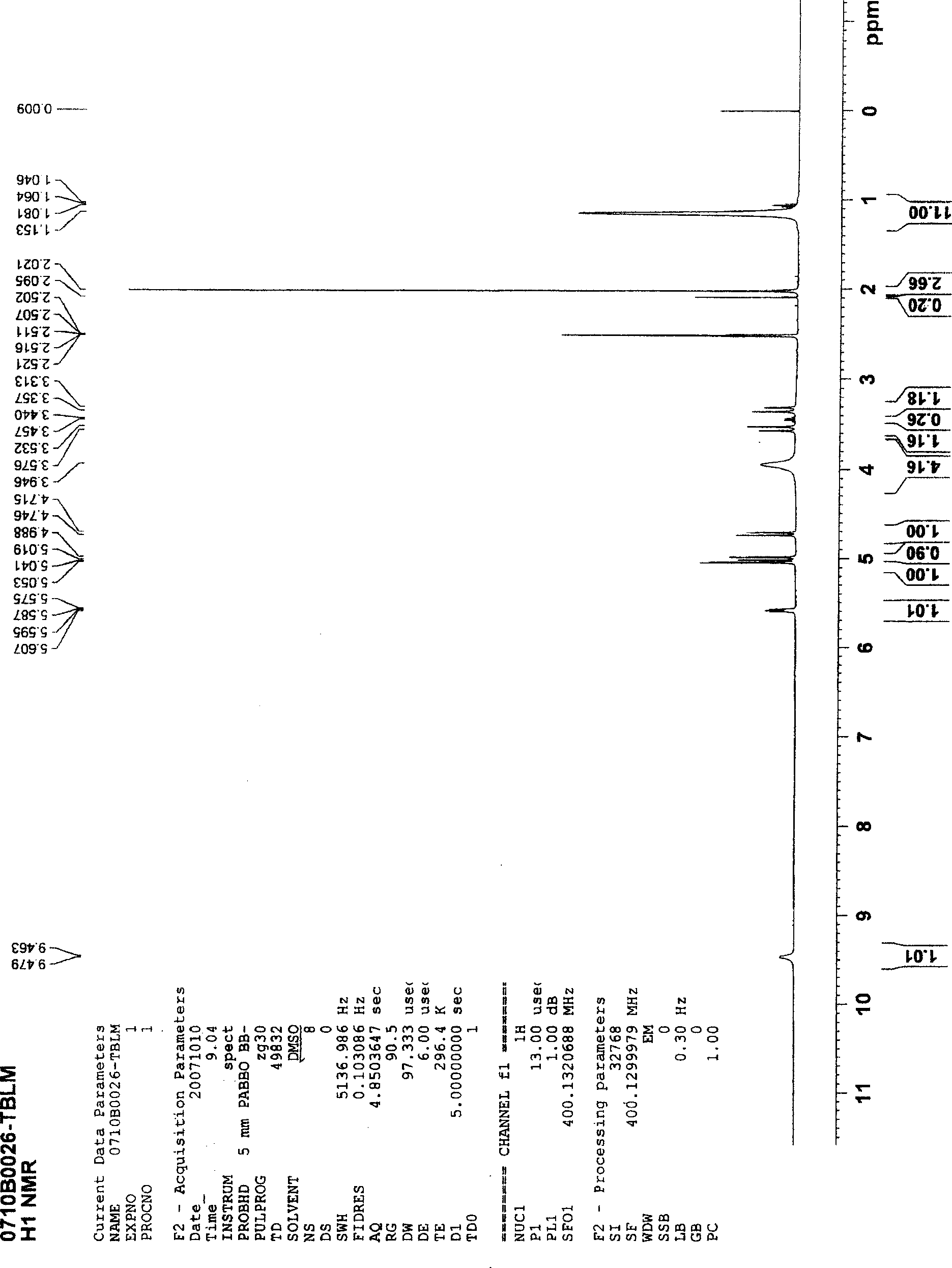
[0029] Add 50 ml of THF and 50 ml of DMA solvent into the reaction vessel, add 100 g of chloroacetyl 7-ACA, 50 g of diisopropylthiourea and 2 g of sodium iodide. React at 30-35°C for 2 hours, add 200 ml of dichloromethane dropwise at 10-15°C, stir for 0.5 hours, add 1000 ml of acetone dropwise at 0-5°C, and the crude cefathiamidine is precipitated with a yield of 103-108 %. Recrystallize and refine with 95% ethanol to obtain a qualified product, the refined yield is 74-78%, and the average total yield is 80%. The content of cefathiamidine measured by HPLC method is greater than 97%, and the color is measured by colorimetry. Lower than the No. 2 yellow standard control solution, and other indicators all meet the quality standards of cefathiamidine on page 156 of the Chinese Pharmacopoeia of the 2005 edition. The nuclear magnetic resonance spectrum of the refined cefathiamidine is as follows figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com