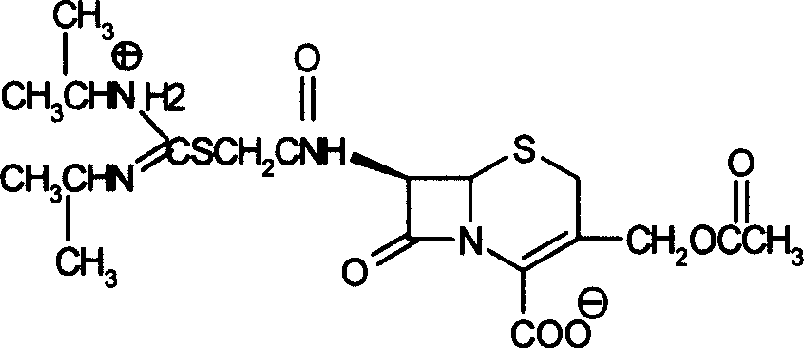
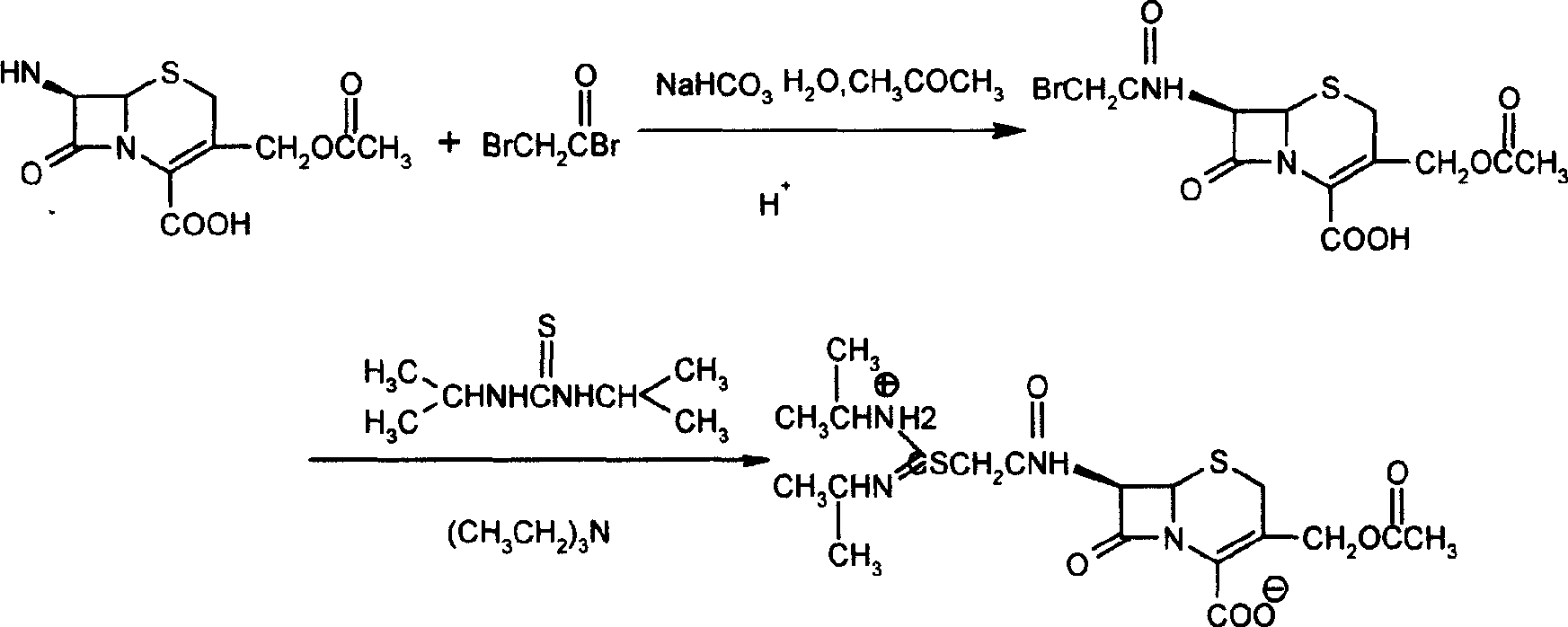
Method for preparing cefathiamidide
A technology for cefathiamidine and cephalosporins is applied in the field of preparation of chemical raw material cefathiamidine, and can solve problems such as being difficult to realize, complicated and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1, cefathiamic acid
[0019] 2.72g 7-ACA, 2.52g NaHCO 3 , 20ml H 2 O and 10ml of acetone were added into a three-necked flask, and stirred at room temperature (32° C.) until dissolved. Then the mixed solution was cooled to about -10°C, and 2.3 g of bromoacetyl bromide (dissolved in 10 ml of acetone) was slowly added dropwise. It took about 1.5 hrs, preferably no solids appeared. After the dropwise addition was completed, the reaction was continued at this temperature (25 D =+120°.
Embodiment 2
[0020] Embodiment 2, cefathiamidine finished product
[0021] Diisopropylthiourea was recrystallized.
[0022] Add 3.93g of cefathiamic acid and 50ml of dichloromethane into a 100ml three-necked flask, slowly add triethylamine to make it just clear (about 1.3ml), add 1.6g of diisopropylthiourea, stir at room temperature for 0.5h, the solution It becomes viscous, slowly add 50ml of acetone dropwise, crystallization begins to precipitate, continue to stir for 0.5h, filter, and wash with acetone. Vacuum dry. Obtain 3.41g of sulfur rice crude product. The yield is 75%, and the melting point is 148-150°C. Optical rotation value: [α] 25 D =139°. The product can be recrystallized from ethanol.
[0023] Elemental Analysis (C 19 h 28 N 4 O 6 S 2 ): C, 46.66; H, 6.41; N, 11.59.
[0024] IR (cm -1 ): 3427.54, 3225.12, 2979.67, 2938.91, 1773.98, 1737.52. m / z: 473, 413, 354, 275, 242, 201.
[0025] 1 HNMR (D 2 O): 5.60-5.59(1H), 5.11-5.10(1H), 4.86-4.83(1H), 4.69-4.66(1H)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com