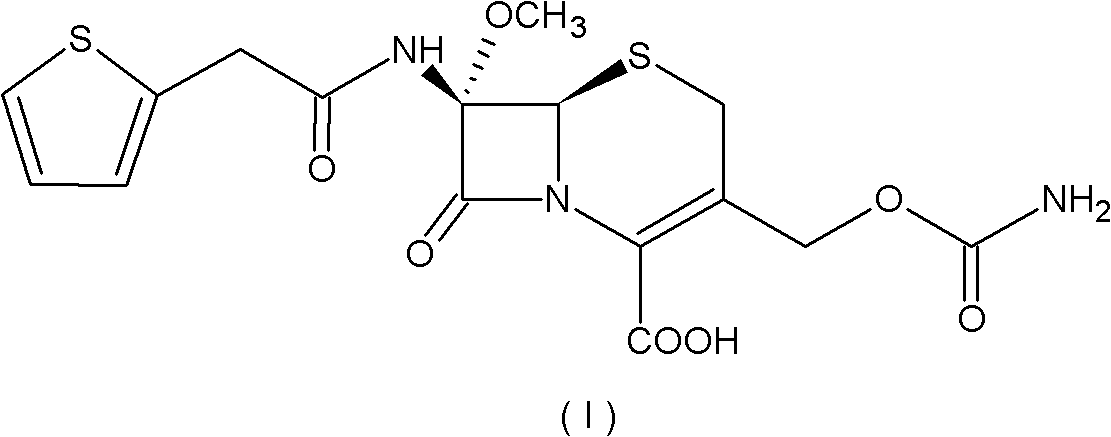
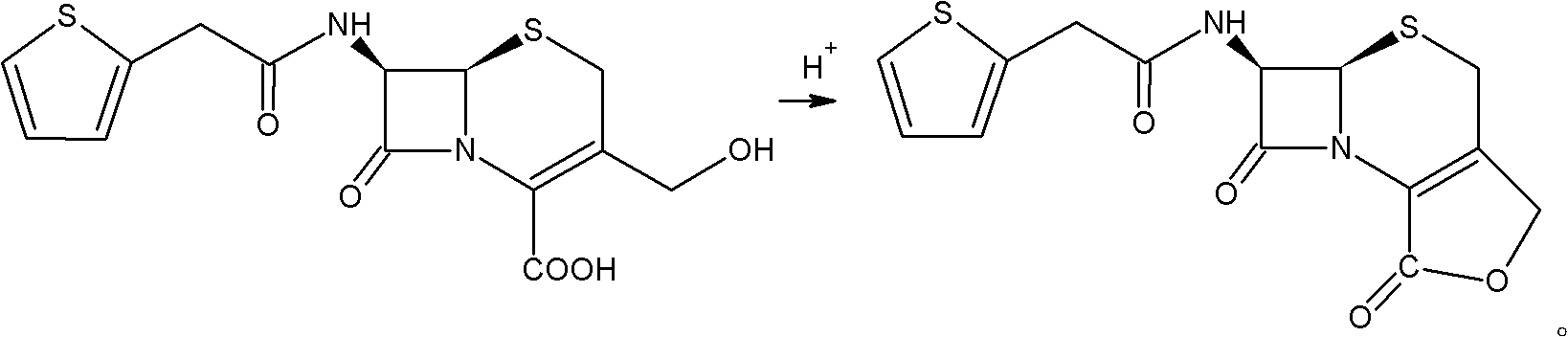
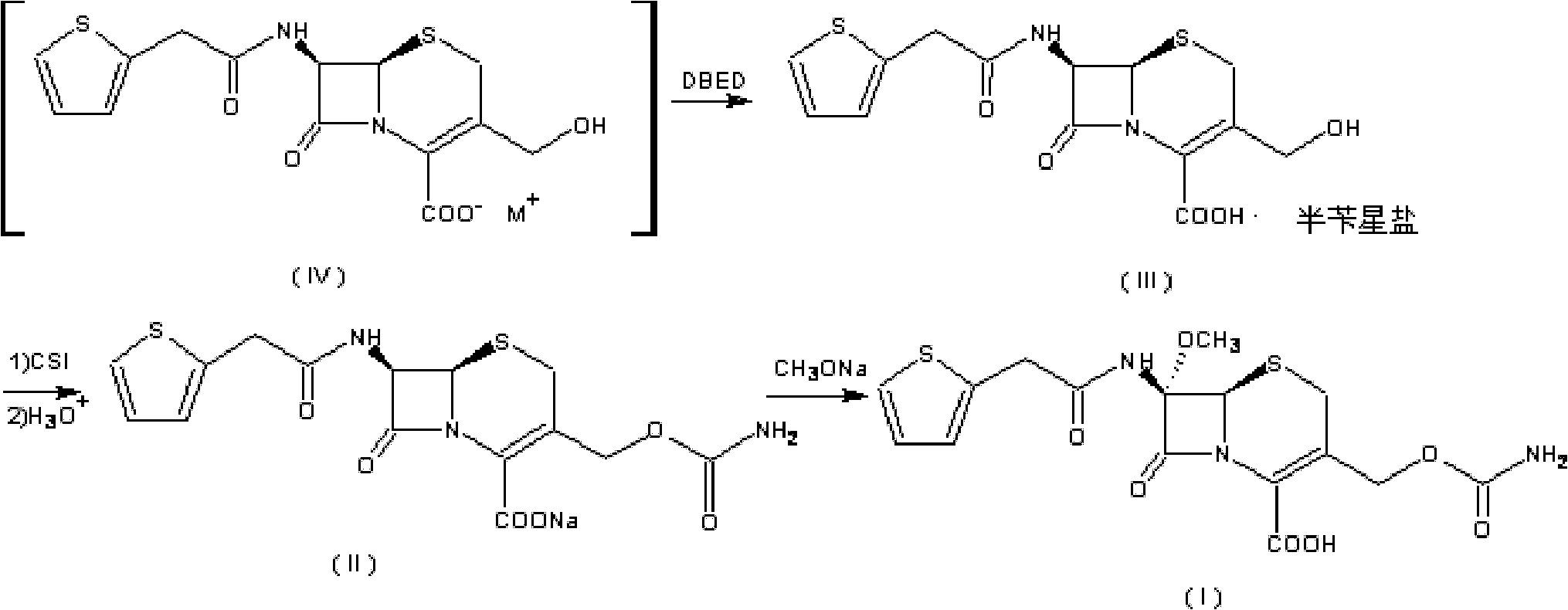
Preparation method of cefoxitin
A cefoxitin and acetyl head technology, applied in the field of pharmaceutical chemical synthesis, can solve the problems of insufficient product quality and yield competitiveness, raw materials or process conditions are not suitable for scale-up production, etc., to achieve easy control of product quality and avoid condensation side effects Effects of Response, Good Product Quality and Yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The preparation method of tert-butyl hypochlorite is synthesized with reference to the synthetic method in "Synthesis and Characterization of New Rubber Chlorinating Agent" (Li Yanli, Shi Shuxian, etc., Journal of Beijing University of Chemical Technology, 31(4):45~49);
[0052] N, N'-dibenzylethylenediamine diacetate was purchased from Shanghai Sinopharm Group Reagent Company, chlorosulfonyl isocyanate was purchased from Shanghai Bangcheng Chemical Company, and methanesulfonic acid was purchased from Shanghai Sinopharm Group Reagent Company.
Embodiment 1
[0054] A preparation method of cefoxitin, comprising the steps of:
[0055] (1) Add 200ml of dichloromethane (organic solvent A) to the aqueous solution 1000ml of the compound of the formula (IV) structure (containing 110g of deacetylcefalotin sodium), and then add 56g of N,N'-dichloromethane at 30°C with temperature control A solution made of benzylethylenediamine diacetate and 800ml of water, the compound of the intermediate formula (III) is precipitated, the temperature is lowered to 0-10°C and stirred for 2 hours; the precipitate is collected by filtration, and the product is washed with water and methylene chloride in turn, Vacuum drying gave 132g of the intermediate compound of formula (III), with a yield of 95.2%;
[0056] through 1 H NMR (400MHz, CDCl 3 ) detection, the results are as follows:
[0057] 1 H NMR (400MHz, CDCl 3 )δ9.02 (d, 1H, J=8.4Hz, CONH), 7.31-7.44 (m, 6H), 6.92-6.96 (m, 2H, thiophene-H), 7.73 (br, 2H, CONH 2 ), 5.53-5.56(dd, 1H), 4.95(d, 1H, J=...
Embodiment 2
[0065] The preparation method of cefoxitin as described in embodiment 1, difference is:
[0066] The organic solvent A described in the step (1) is chloroform, and 128.5 g of the compound of the intermediate formula (III) is obtained, with a yield of 94.9%.
[0067] The compound of intermediate formula (III) structure undergoes 1 H NMR (400MHz, CDCl 3 ) detection, the result is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com