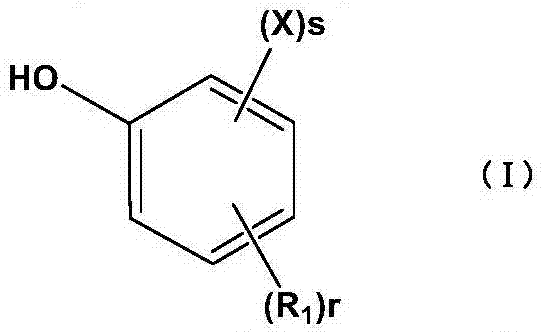
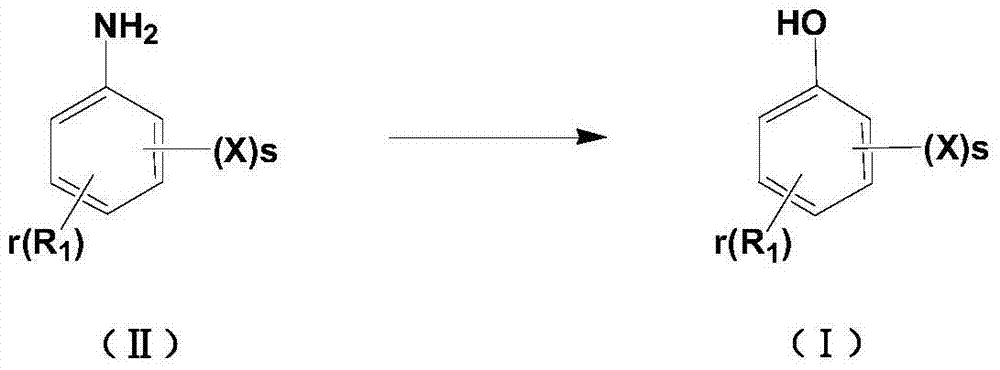
Method for preparing halogenated phenol compounds
A technology of halogenated phenols and compounds, which is applied in the field of preparation of halogenated phenols, can solve the problems of low yield and non-reaction, and achieve the effects of easy separation and treatment, reduced side reactions, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0035] Embodiment 12, the preparation of 5-dichlorophenol
[0036] 32.6g of 2,5-dichloroaniline and 100 mL of 15% hydrochloric acid aqueous solution were added to the reaction flask, heated to 90°C, cooled to 0°C after reaction for 1 hour, and filtered to obtain 2,5-dichloroaniline hydrochloride. The 2,5-dichloroaniline hydrochloride filter cake obtained by filtration was added to the autoclave, and then 100 mL of water, 100 mL of toluene, 0.163 g of phase transfer catalyst, 3.26 g of solid superacid SO were added. 4 2- / ZrO 2 , 150 ℃ closed reaction for 4 hours, cooled to 20 ℃, opened the kettle and discharged, filtered, the filtrate was left to stand for phase separation, the organic phase was distilled to remove toluene, the organic phase was analyzed by gas chromatography, the selectivity was 98%, the conversion rate was 100%, and the pressure was continued. Rectification, the pressure is 266.6Pa (2mm Hg column), the fraction of 68-73 ° C is collected to obtain 31.5g of ...
Embodiment 22
[0037] Example 22, Preparation of 5-dichlorophenol
[0038] Collect the filtrate of 2,5-dichloroaniline hydrochloride prepared in Example 1, add 32.6g of 2,5-dichloroaniline, and add 25g of 30% aqueous hydrochloric acid solution, heat to 90 ° C, and cool to 1 h after the reaction. 0 °C, filtration to obtain 2,5-dichloroaniline hydrochloride. The 2,5-dichloroaniline hydrochloride filter cake obtained by filtration is added in the autoclave, then 100mL of water, 100mL of toluene, 0.163g phase transfer catalyst are added, and the solid superacid SO that is collected by filtration in above-described embodiment 1 is recovered. 4 2- / ZrO 2, 150 ℃ closed reaction for 4 hours, cooled to 20 ℃, opened the kettle and discharged, filtered, the filtrate was left to stand for phase separation, the organic phase was distilled to remove toluene, the organic phase was analyzed by gas chromatography, the selectivity was 97%, the conversion rate was 100%, and the pressure was continued. Recti...
Embodiment 3
[0039] Example 3 Catalytic preparation of 2,5-dichlorophenol using different phase transfer catalysts
[0040] 32.6g of 2,5-dichloroaniline and 100 mL of 15% hydrochloric acid aqueous solution were added to the reaction flask, heated to 90°C, cooled to 0°C after reaction for 1 hour, and filtered to obtain 2,5-dichloroaniline hydrochloride. Add 2,5-dichloroaniline hydrochloride, 100mL water, 100mL toluene, phase transfer catalyst, 3.26g solid super acid SO to the autoclave 4 2- / ZrO 2 , 150 ℃ closed reaction for 4 hours, cooled to 20 ℃, opened the kettle and discharged, filtered, the filtrate was left to stand for phase separation, the organic phase was distilled to remove toluene, the organic phase was analyzed by gas chromatography, and the vacuum distillation was continued, the pressure was 266.6Pa (2mm Hg column), the 68-73 °C fractions were collected to give 2,5-dichlorophenol (see Table 1).
[0041] Table 1
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com