Antibacterial drugs cefoxitin preparation process
A technology of cefoxitin and cefoxitin sodium, which is applied in the direction of organic chemistry, can solve the problems of high production cost and low yield, achieve cost saving, increase reaction yield, and solve the effects of low reaction yield
Active Publication Date: 2010-12-08
SHANDONG SALUBRIS PHARMA +1
View PDF3 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The actual production has very strict requirements on the reaction conditions, and the production cost is high
Orchid company (Deshpande et al., WO2004083217) omits the protection and deprotection of carboxyl group, directly uses sodium methylate or lithium methylate etc. as strong base, and uses NCS or tert-butyl hypochlorite etc. as reagents at -90°C. The 7-position introduces a methoxyl group, and obtains II in one step, but the yield is low
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
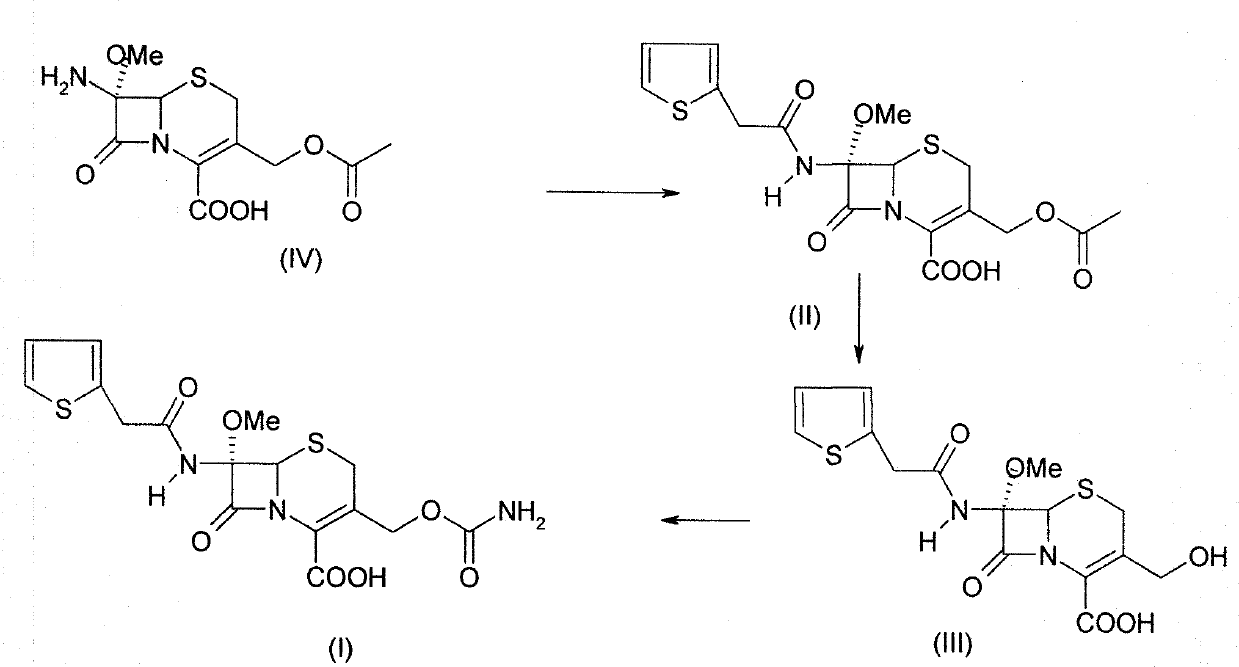
The invention provides a method for preparing cefoxitin (I). It comprises taking 7 alpha- methoxy- 7 beta- aminocephalosporanic acid (7- MAC) as raw material, carrying out 2- thiofuran acetylation reaction, hydrolytic reaction, carbamylation reaction and getting cefoxitin (I). The invention greatly increases the prductivity for cefoxitin and its sodium salt and reduces production cost.
Description
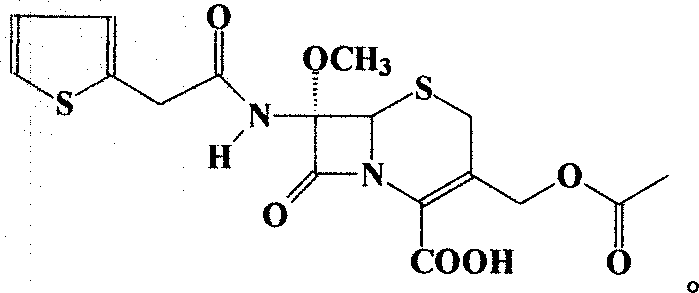
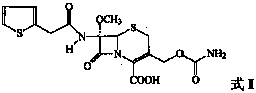
technical field The invention relates to a preparation method of a compound, in particular to a preparation method of a cephalosporin. Background technique Cefoxitin (Cefoxitin,), the chemical name is (6R, 7S)-3-carbamoyloxymethyl-7-methoxy-8-oxo-7-[2-(2-thienyl)acetamido ]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid is a semi-synthetic cephamycin antibiotic developed by Merck Company of the United States. Its chemical structural formula (I) is as follows: Clinically, the sodium salt of cefoxitin is used to make injections for the treatment of bacterial infections. The drug was first approved for marketing by Japan's Daiichi Pharmaceutical Development Company in August 1979 under the trade name Cenomycin. The antibacterial effect of cefoxitin is similar to that of the second-generation cephalosporins, but because its structure contains a 7α-methoxy group, it greatly reduces the hydrolytic damage of β-lactamase in bacteria, and its drug resistance Much less o...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D501/34
Inventor 谭端明张黎辉叶澄海
Owner SHANDONG SALUBRIS PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com