Process for producing 7-alpha-methoxy-3-deacetyled cefoxitin benzathine
A technology of thiophene benzyl star salt and acetyl head, which is applied in the field of preparation of 7-α-methoxy-3-deacetyl cefotaxime benzyl star salt, and achieves mild reaction conditions, reduced production cost and reduced emission to the environment. Contamination, avoid the effect of low temperature requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
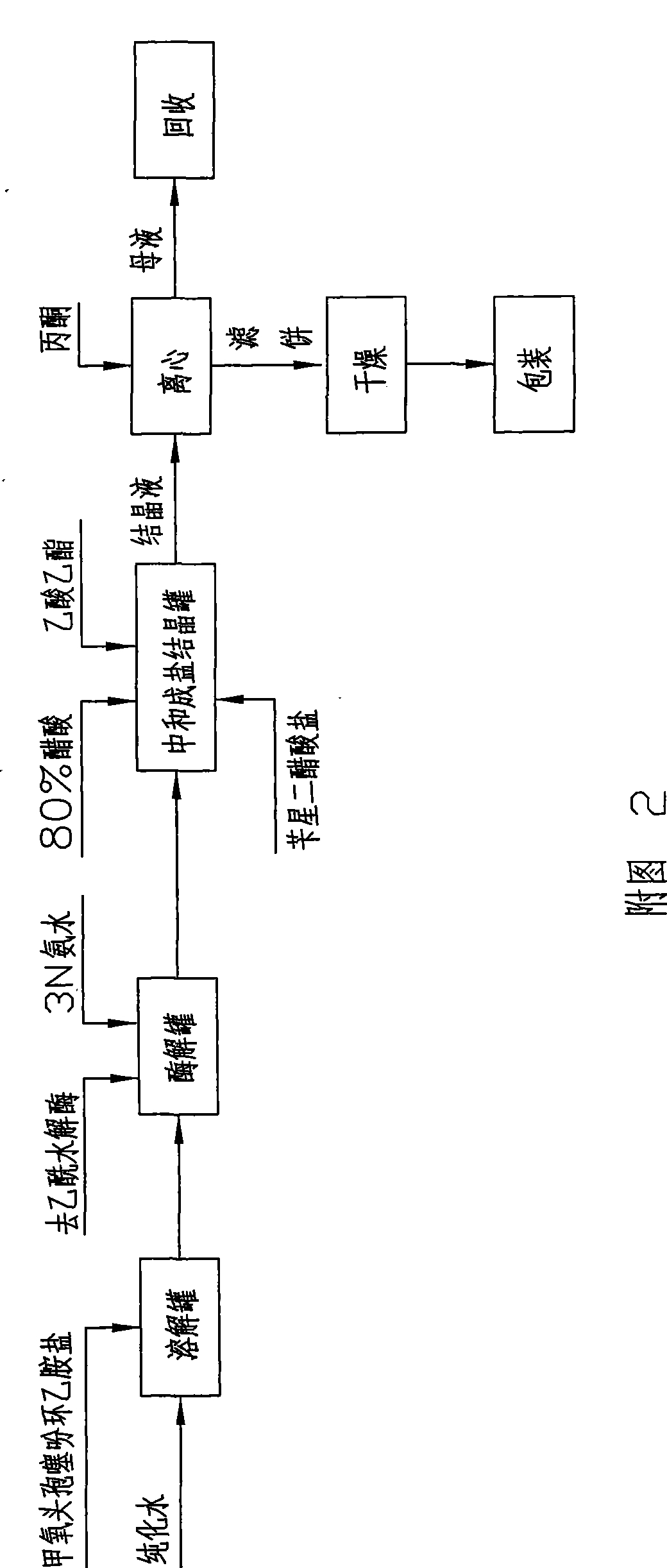
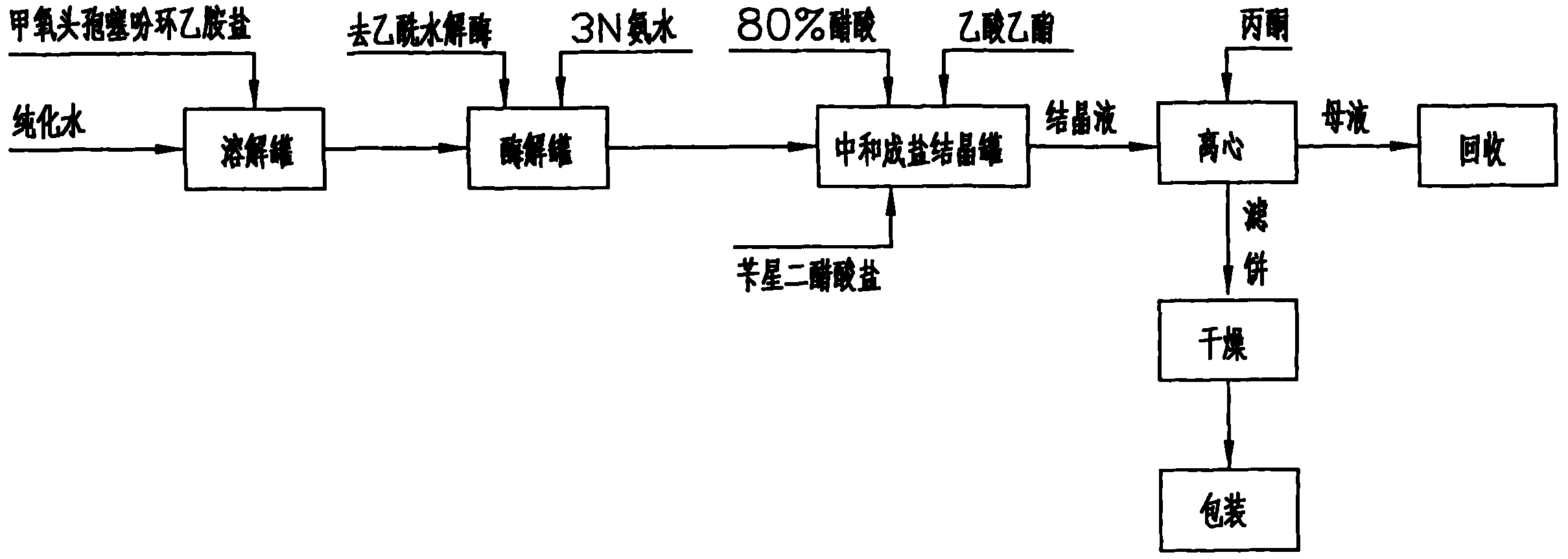
[0035] Embodiment 1: As shown in Figure 2, the preparation method of 7-alpha-methoxy-3-deacetyl cephalothin benzathine salt comprises the following steps:
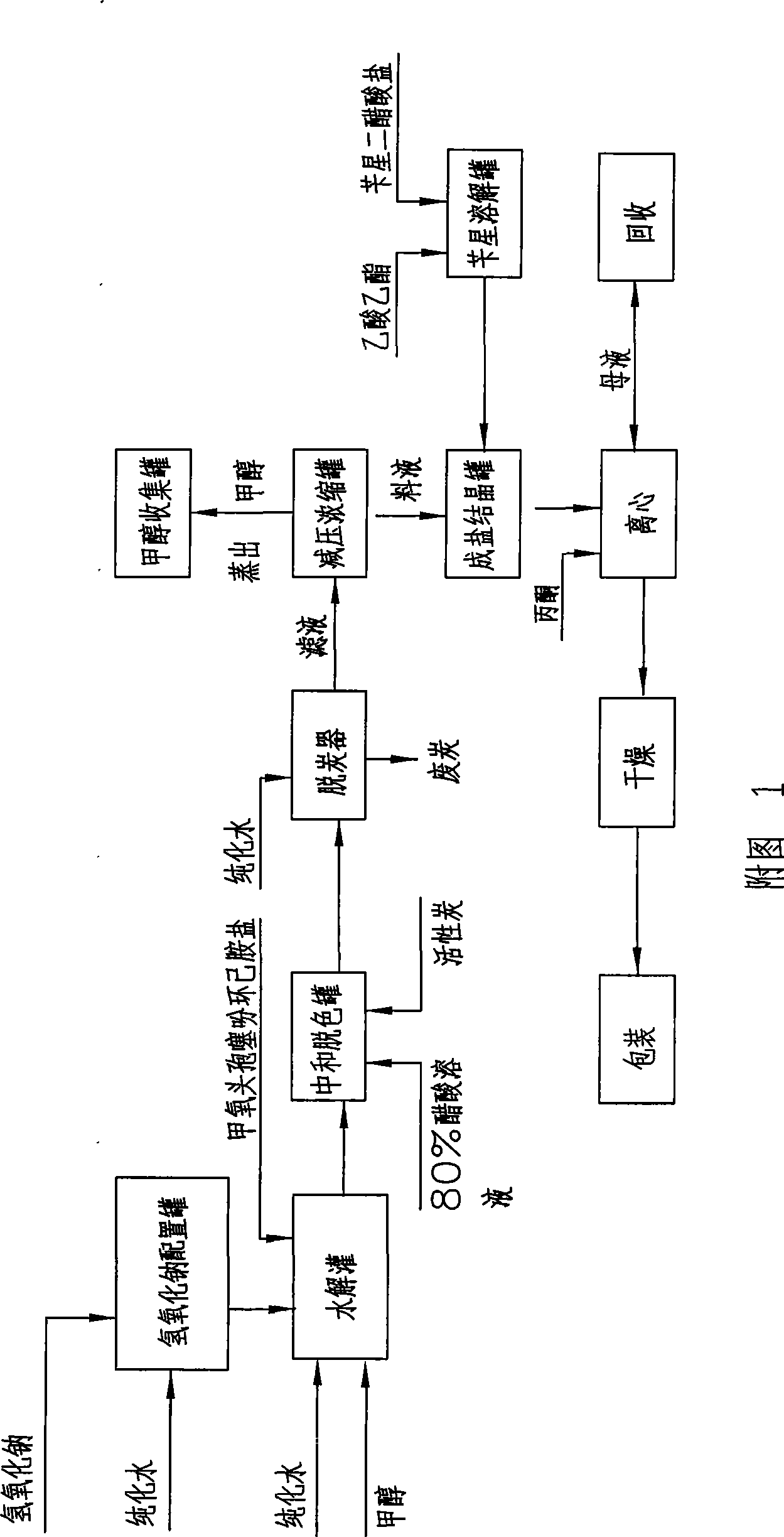
[0036] a. Enzymolysis: Add 100Kg of 7-α-methoxycefalotin cyclohexylamine salt into 500 liters of purified water, stir to dissolve it completely, adjust the pH to 6.5-8.0 with 1N ammonia water, and pour the material into the immobilized After the enzymolysis tank of deacetylase (wherein the immobilized deacetylase is produced by the research institute of Hebei Jiupai Pharmaceutical Co., Ltd., the batch number is: ym080906-160, and the addition amount is 15Kg), control the solution temperature at 20-25°C, Under stirring state, control the pH value at 7.0 to carry out the enzymolysis reaction until the pH does not change within 10 minutes, and when the residual 7-α-methoxycefalotin cyclohexylamine salt is detected by sampling and ≤ 1.0%, the enzymolysis is completed;
[0037] b. Neutralization: after the above-mentioned enzym...
Embodiment 2
[0040] a. Enzyme hydrolysis: Add 100Kg of 7-α-methoxycefalotin cyclohexylamine salt into 1000 liters of purified water, stir to make it completely dissolved, adjust the pH to 6.5-8.0 with 5N sodium carbonate solution, and pour the material into the After the enzymolysis tank of the immobilized deacetylase (wherein the immobilized deacetylase is produced by the Research Institute of Hebei Jiupai Pharmaceutical Co., Ltd., the batch number is ym080906-160, and the addition amount is 50Kg), the temperature of the solution is controlled at 20-25°C, Under stirring, control the pH value at 8.0 to carry out the enzymolysis reaction until the pH does not change within 10 minutes, and when the residual 7-α-methoxycefalotin cyclohexylamine salt is detected by sampling and ≤ 1.0%, the enzymolysis is complete;
[0041] b. Neutralization: After the above-mentioned enzymolysis reaction is completed, put the reacted enzymolysis fluid into the crystallization tank, and then use a small amount o...
Embodiment 3
[0044] a. Enzymolysis: Add 100Kg of 7-α-methoxycefalotin cyclohexylamine salt into 750 liters of purified water, stir to make it completely dissolve, adjust the pH to 6.5-8.0 with 5N sodium carbonate solution, and pour the material into the After the enzymolysis tank of immobilized deacetylase (wherein the immobilized deacetylase is produced by Hebei Jiupai Pharmaceutical Co., Ltd. Research Institute, the batch number is ym080906-160, and the addition amount is 75Kg), control the solution temperature at 20-25°C, Under stirring, control the pH value at 8.0 to carry out the enzymolysis reaction until the pH does not change within 10 minutes, and when the residual 7-α-methoxycefalotin cyclohexylamine salt is detected by sampling and ≤ 1.0%, the enzymolysis is complete;
[0045] b. Neutralization: After the above-mentioned enzymolysis reaction is completed, put the reacted enzymolysis fluid into the crystallization tank, and then use a small amount of 80% acetic acid to adjust the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com