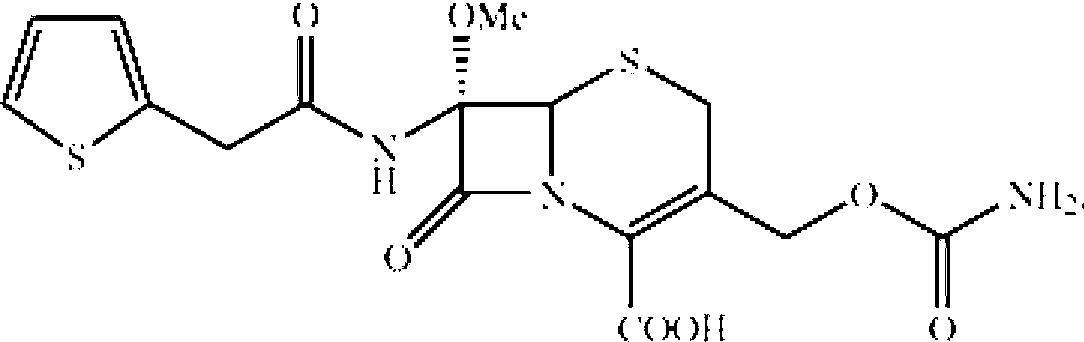
Method for preparing cefoxitin acid as antibacterial medicament
A technology for cefoxitin and antibacterial drugs, applied in the field of preparation of antibacterial drug cefoxitin, can solve the problems of requiring precious metal catalysts, high cost, difficult to obtain, etc. The effect of small particle size of synthetic powder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A preparation method of antibacterial drug cefoxitin, comprising the steps of:
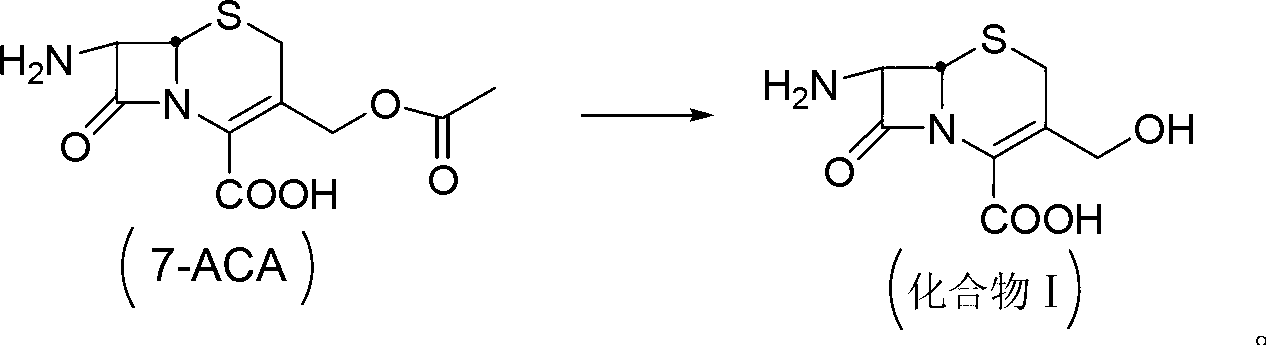
[0046] Preparation of deacetyl 7-aminocephalosporanic acid
[0047] Add 60g of 7-ACA into the reaction vessel, disperse in 350mL of water, add 25% sodium hydroxide solution, adjust the pH of the solution to 8.1, control the temperature at 5°C for hydrolysis reaction, continue to stir for 45min after dissolving, and add ethyl acetate 125mL, stirred for 15min, then added dropwise 1.2mol / L dilute hydrochloric acid to adjust the pH to 2.7, precipitated crystals, and dried to obtain 49.9g of deacetylated 7-aminocephalosporanic acid, with a yield of 98.34%;
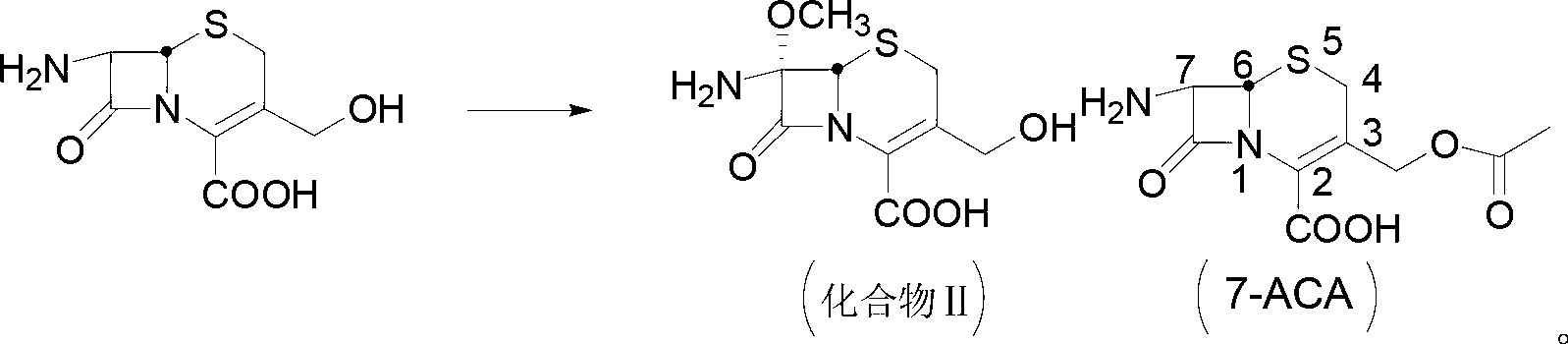
[0048] Preparation of compound Ⅱ
[0049] Add 49.9g of compound I into a mixture of 360mL of dichloromethane and 30mL of methanol, cool down to -23°C, and stir for 20min. Add 2.082g of methanesulfonic acid, cool to -55°C, add 77.15g of NBS in batches, add 292.55g of sodium methoxide, stir thoroughly for 3h, take samples every 0.5h by high-...
Embodiment 2
[0055] A preparation method of antibacterial drug cefoxitin, comprising the steps of:
[0056] Preparation of deacetyl 7-aminocephalosporanic acid
[0057] Add 60g of 7-ACA to the reaction vessel, disperse it in 350mL of water, add 20% sodium hydroxide solution, adjust the pH of the solution to 8.2, control the temperature at 2°C for hydrolysis reaction, continue to stir for 60min after dissolving, Add 125 mL of ethyl acetate, stir for 15 min, then add 1.2 mol / L dilute hydrochloric acid dropwise to adjust the pH=3, crystals are precipitated, and dried to obtain 50.1 g of deacetyl 7-aminocephalosporanic acid with a yield of 98.73%.
[0058] Preparation of compound Ⅱ
[0059] Add 50.01g of compound I into a mixture of 300mL of dichloromethane and 36mL of methanol, cool down to -25°C, and stir for 20min. Add 2.091g of methanesulfonic acid, cool to -60°C, add 39.01g of NBS in batches, add 352.2g of sodium methoxide, stir thoroughly for 6h, take samples every 0.5h by high perform...
Embodiment 3
[0065] A preparation method of antibacterial drug cefoxitin, comprising the steps of:
[0066] Preparation of deacetyl 7-aminocephalosporanic acid
[0067] Add 60g of 7-ACA to the reaction vessel, disperse it in 350mL of water, add 30% sodium hydroxide solution, adjust the pH of the solution to 8.4, control the temperature at 4°C for hydrolysis reaction, continue to stir for 30min after dissolving, add Ethyl acetate 125mL, stirred for 15min, then dilute hydrochloric acid (1.2mol / L) was added dropwise to adjust the pH=3.4, crystals were precipitated, and dried to obtain 50.3g of deacetylated 7-aminocephalosporanic acid, with a yield of 99.12%;
[0068] Preparation of compound Ⅱ
[0069] Add 50.3g of compound I into a mixture of 360mL of dichloromethane and 36mL of methanol, cool down to -20°C, and stir for 20min. Add 2.099g of methanesulfonic acid, cool to -55°C, add 59.22g of NBS in batches, add 294.84 (401.45) g of sodium methoxide, stir well for 4 hours, and take samples e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com