Method for producing cefoxitin acid
A technology of cefoxitin and lactam, which is applied in the field of preparation of cefoxitin, can solve the problems of high operating conditions and cost requirements, difficult to store, and high operating conditions, and achieves that the reagents are easy to obtain and store, easy to separate and purify, and The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
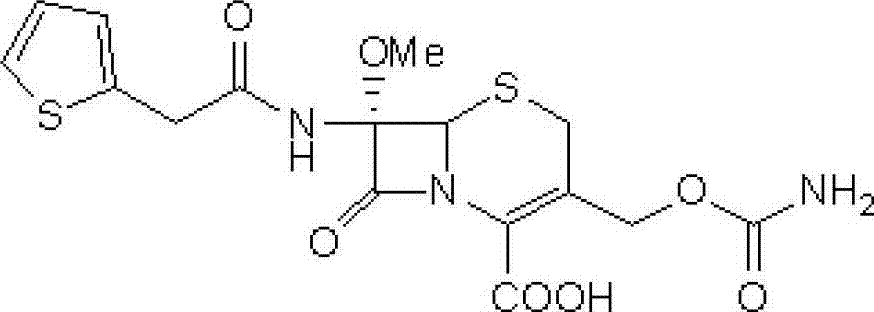
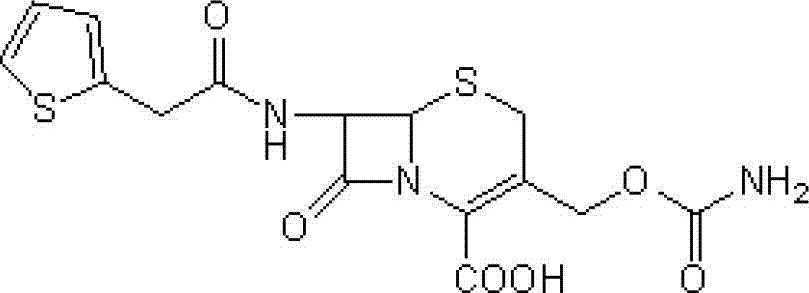
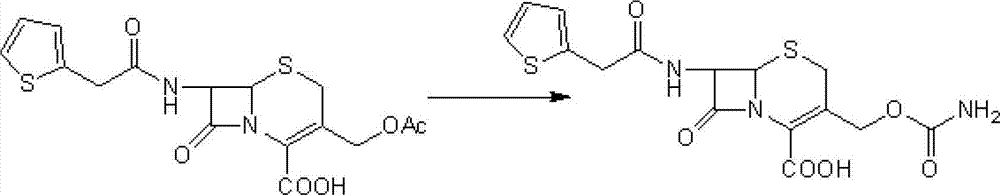
[0036] Step 1: Intermediate (6R,7S)-3-carbamoyloxymethyl-7-[2-(2-thienyl)acetamido]-8-oxo-5-thia-1-aza Preparation of bicyclo[4.2.0]oct-2-ene-2-carboxylic acid
[0037] Add 100 g of cephalothin to 500 ml of diethyl ether, add 350 ml of triethylamine and 54 g of chlorosulfonyl isocyanate, stir at -10°C for 8 hours, then add 0.75 L of 97% ethanol, stir at 25°C for 40 minutes, and concentrate the reaction solution under reduced pressure Then, it was added to ice water at 0°C and stirred for 30 minutes. After suction filtration, the filter cake was washed with ice water at 0°C and dried in vacuo to obtain 90 g of a light yellow solid (the intermediate), yield: 89.8%.
[0038] Second step: the preparation of cefoxitin
[0039] Add 85g of the intermediate obtained in the first step and 400ml of anhydrous methanol into the dry reactor, stir at 25°C for 30 minutes, then cool to -50°C, add 54.2g of powdered sodium methoxide, keep stirring for 2 hours, then add 100ml of 5% hydrochlori...
Embodiment 2
[0041] Step 1: Intermediate (6R,7S)-3-carbamoyloxymethyl-7-[2-(2-thienyl)acetamido]-8-oxo-5-thia-1-aza Preparation of bicyclo[4.2.0]oct-2-ene-2-carboxylic acid
[0042] Add 100 g of cephalothin to 500 ml of petroleum ether, add 350 ml of triethylamine and 54 g of trimethylsilyl isocyanate, stir and react at 5°C for 12 hours, then add 0.75 L of 97% ethanol, and stir at 25°C for 40 minutes. The reaction solution was concentrated under reduced pressure, added to ice water at 0°C, and stirred for 30 minutes. After suction filtration, the filter cake was washed with ice water at 0°C and dried in vacuo to obtain 87.2 g of a light yellow solid (the intermediate), yield: 87%.
[0043] Second step: the preparation of cefoxitin
[0044] Add 85g of the intermediate obtained in the first step, 400ml of anhydrous tetrahydrofuran into the drying reactor, stir at 25°C for 30 minutes, then cool to -5°C, add 54.2g of powdered sodium methoxide, keep stirring for 2 hours, then add 100ml of 5% ...
Embodiment 3
[0046] Step 1: Intermediate (6R,7S)-3-carbamoyloxymethyl-7-[2-(2-thienyl)acetamido]-8-oxo-5-thia-1-aza Preparation of bicyclo[4.2.0]oct-2-ene-2-carboxylic acid
[0047] Add 100 g of cephalothin to 500 ml of ethyl acetate, add 350 ml of triethylamine and 54 g of dichlorophosphorinyl isocyanate, stir and react at 0°C for 10 hours, then add 0.75 L of 97% ethanol, and stir at 25°C for 40 minutes. The reaction solution was concentrated under reduced pressure, added to ice water at 0°C, and stirred for 30 minutes. Filter with suction, and wash the filter cake with ice water at 0°C. Dry in vacuo to obtain 87.8 g of light yellow solid (ie intermediate), yield: 87.6%.
[0048] Second step: the preparation of cefoxitin
[0049] Add 85g of the intermediate obtained in the first step and 400ml of anhydrous methanol into the drying reactor, stir at 25°C for 30 minutes, then cool to -30°C, add methanol-sodium methoxide solution containing 54.2g of sodium methoxide, and keep stirring for 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com