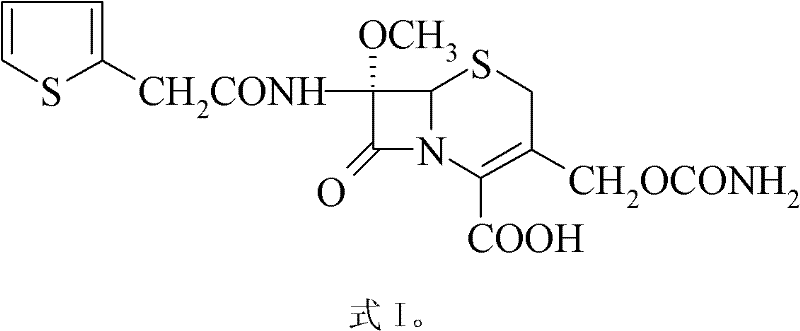
A kind of cefoxitin compound and composition thereof
A technology of cefoxitin and compounds, which is applied in the field of cefoxitin compounds and their compositions, can solve problems such as high price, and achieve the effects of improving drug safety, good dissolution stability, and excellent dissolution performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
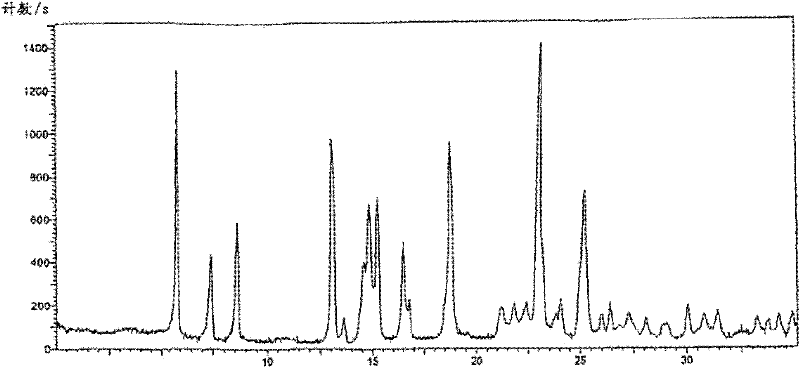
[0058] The preparation of described cefoxitin compound is: get 100g of cefoxitin crude product, add the volume ratio that is 7.5:2.5 methanol: ethanol solution that volume is 8 times of cefoxitin crude product weight, heat to reflux; Cefoxitin crude product dissolves clear Finally, add 0.5 times of activated carbon for decolorization for 30 minutes, filter; the filtrate stops heating, naturally cools down to 43 ° C, maintains the temperature and adds dropwise acetone with a volume ratio of 5 times the weight of the crude cefoxitin: aqueous solution of 9: 1, the resulting The dropwise addition is at a constant speed of 6 to 7 minutes at a stirring speed of 12 to 14rmp; after dropping, stir and cool down, the stirring and cooling is to cool down to room temperature for 10 minutes under stirring at a rotating speed of 17 to 18rmp, and then stir at a rotating speed of 11rmp for 20min Cool down to 12-13°C, let stand for 19 hours, filter, wash twice with 8:2 methanol:water solution, ...
Embodiment 2
[0061] The preparation of described cefoxitin compound is: get 100g of cefoxitin crude product, add the volume ratio that is 7.5:2.5 methanol: ethanol solution that volume is 8 times of cefoxitin crude product weight, heat to reflux; Cefoxitin crude product dissolves clear Finally, add 0.5 times of activated carbon for decolorization for 30 minutes, filter; the filtrate stops heating, naturally cools down to 43 ° C, maintains the temperature and adds dropwise acetone with a volume ratio of 5 times the weight of the crude cefoxitin: aqueous solution of 9: 1, the resulting The dropwise addition is at a stirring speed of 11 to 13rmp, and is added dropwise at a constant speed within 5 minutes; after dropping, stir and cool down. 11~12 ℃, stand still for 18 hours, filter, with 8: 2 methanol: aqueous solution washes 2 times, each dosage is 0.4 times of the volume of cefoxitin crude product weight, dry, obtain described cefoxitin compound, yield Yield 91.2%, HPLC content 99.52%, mp.1...
Embodiment 3
[0064] The preparation of described cefoxitin compound is: get 100g of cefoxitin crude product, add the volume ratio that is 7.5:2.5 methanol: ethanol solution that volume is 8 times of cefoxitin crude product weight, heat to reflux; Cefoxitin crude product dissolves clear Finally, add 0.3 times of activated carbon for decolorization for 30 minutes, filter; the filtrate stops heating, naturally cools down to 42 ° C, maintains the temperature and adds dropwise acetone with a volume ratio of 4 times the weight of the crude cefoxitin: aqueous solution of 9: 1, the resulting The dropwise addition is at a constant speed of 7 to 8 minutes at a stirring speed of 12 to 14rmp; after dropping, stir and cool down, the stirring and cooling is to cool down to room temperature for 10 minutes under stirring at a rotating speed of 15 to 17rmp, and then stir at a rotating speed of 10rmp for 20min Cool down to 10-11°C, let stand for 19 hours, filter, wash 3 times with 8:2 methanol:water solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com