Cefoxitin acid preparation method
A technology of cefoxitin acid and acetyl head, which is applied in the field of preparation of cefoxitin acid, can solve the problems that tetrahydrofuran cannot be reused, reduces the yield of final products, and the cost of cefoxitin acid is high, and achieves significant social and economic benefits. Effects of recycling and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
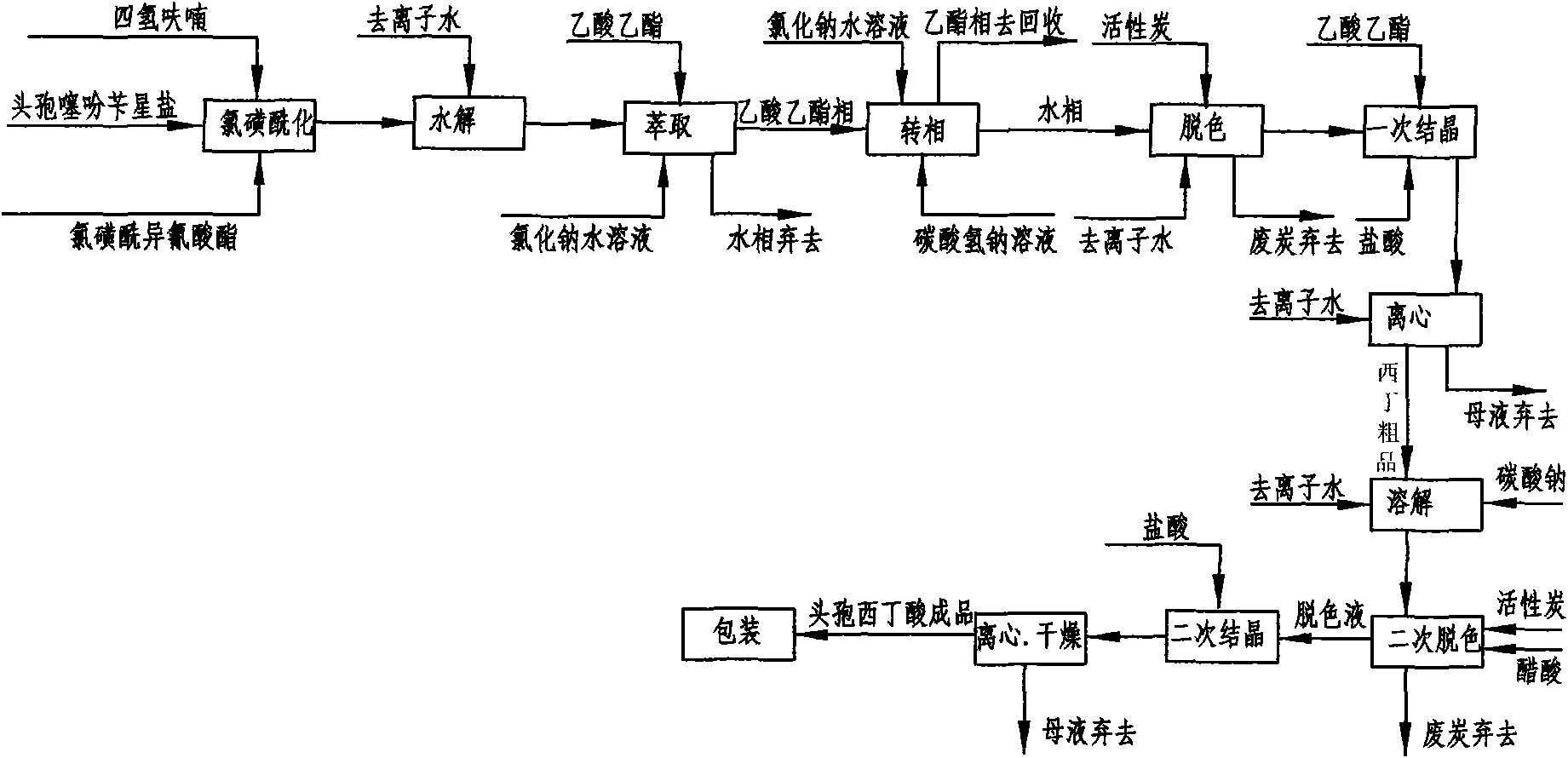
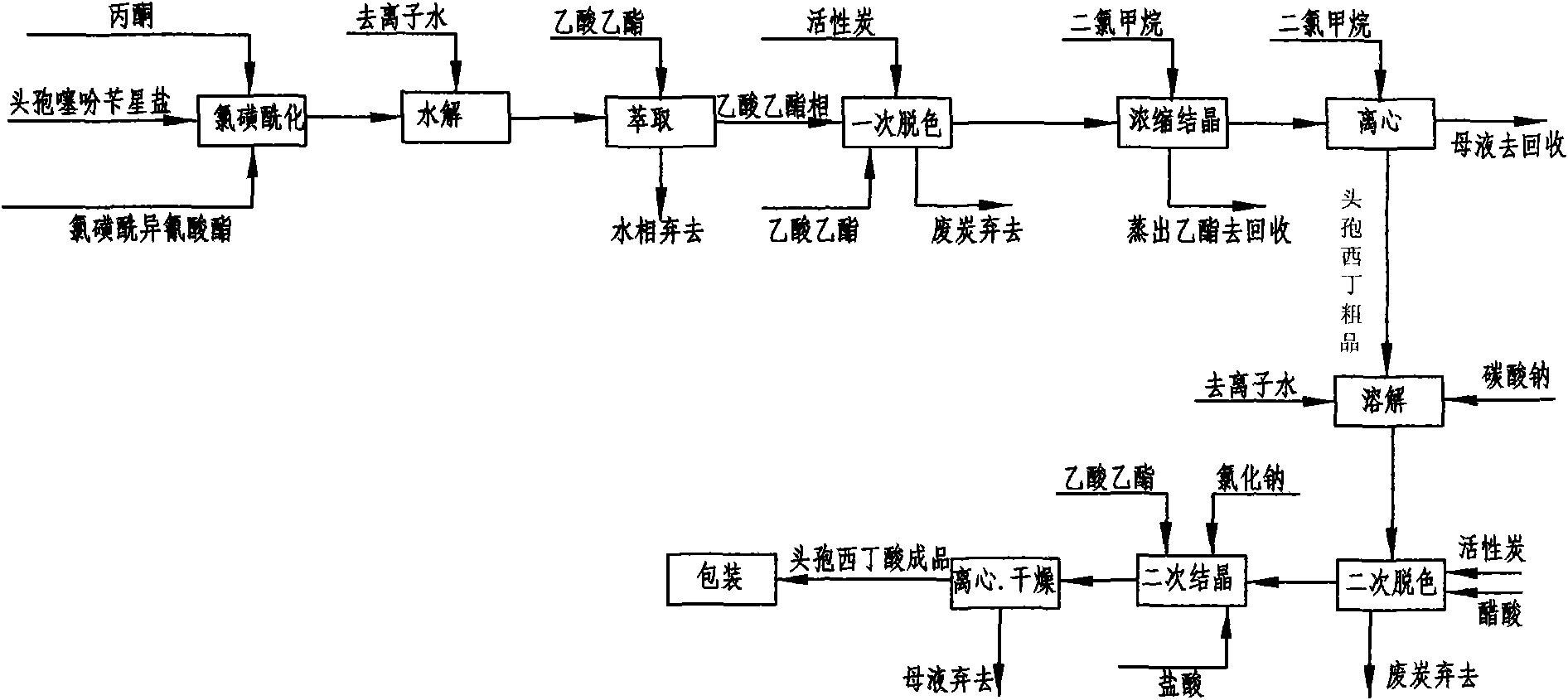
[0043] Embodiment 1: as figure 2 Shown, this is that reaction solvent, 7-alpha-methoxy-3-deacetyl cephalothin benzine salt is the method for starting raw material preparation cefoxitin acid by acetone and comprises the following steps:
[0044] a. Chlorosulfonylation: Add 50 g of 7-α-methoxy-3-deacetyl cephalothin benzathine salt to 300 ml of acetone at room temperature 25°C, and add chlorosulfonyl when cooled to -50~-60°C 26g of isocyanate, under the stirring state, use liquid nitrogen to control the temperature to react at -60°C ~ -30°C, keep stirring for 60 minutes and then take a sample for detection. When the initial raw material 7-α-methoxy-3-deacetyl cephalothin End reaction when star salt ≤ 1.0%;
[0045] b. Hydrolysis: Pour the reaction solution after the above reaction into 100ml deionized water, stir, use cold salt water to control the temperature at 0-20°C to carry out the hydrolysis reaction, keep stirring for 60 minutes and then take a sample for detection. Whe...
Embodiment 2
[0052] Embodiment 2: the difference between this embodiment and embodiment 1 is:
[0053] a. Chlorosulfonylation: at room temperature 25°C, add 50g of 7-α-methoxy-3-deacetylcephalothin benzathine salt to 500ml of acetone, add chlorosulfonyl when cooled to -50~-60°C 35g of isocyanate, under stirring, use liquid nitrogen to control the temperature to react at -60°C ~ -30°C, take a sample for detection, when the initial raw material 7-α-methoxy-3-deacetyl cephalothin benzathine salt ≤ 1.0% end the reaction;
[0054] b. Hydrolysis: Pour the reaction solution after the above reaction into 100ml deionized water, stir, and use cold salt water to control the temperature at 0-20°C to carry out the hydrolysis reaction, keep stirring for 60 minutes and then take a sample for detection. When the intermediate N- The reaction ends when the chlorosulfonic acid derivative is ≤1.0%;
[0055] c. Extraction: Add 600ml of ethyl acetate to the above hydrolyzate, stir for 10 minutes and then filt...
Embodiment 3
[0061] Embodiment 3: the differences between this embodiment and embodiment 1 are:
[0062] a. Chlorosulfonylation: at room temperature 25°C, add 50g of 7-α-methoxy-3-deacetylcephalothin benzathine salt to 450ml of acetone, add chlorosulfonyl when cooled to -50~-60°C 40g of isocyanate, under stirring, use liquid nitrogen to control the temperature to react at -60°C ~ -30°C, take a sample for detection, when the initial raw material 7-α-methoxy-3-deacetyl cephalothin benzathine salt ≤ 1.0% end the reaction;
[0063] b. Hydrolysis: Pour the above-mentioned reaction solution into 100ml deionized water, stir, and use cold salt water to control the temperature at about 10°C to carry out the hydrolysis reaction. After stirring for 60 minutes, take a sample to detect when the intermediate N-chlorosulfur The reaction ends when the acid derivative is ≤1.0%;
[0064] c. Extraction: Add 800 ml of ethyl acetate to the above hydrolyzate, stir for 10 minutes and then filter. The filtrate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com