Method for preparing tembotrions
A technology of cyclosulfonone and cyclohexanedione is applied in the field of preparation of cyclosulfonone, can solve the problems of low yield of cyclosulfonone, contains highly toxic substances, and high cost, and achieves high reaction yield, easy operation, and high cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A kind of preparation method of tembotrione provided by the present embodiment comprises the following steps:
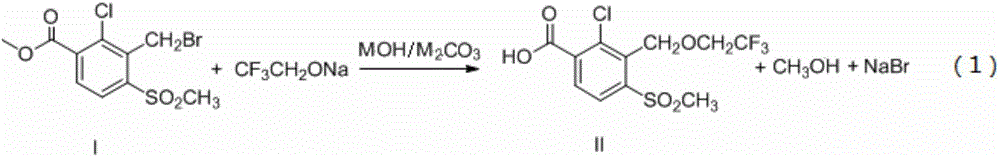
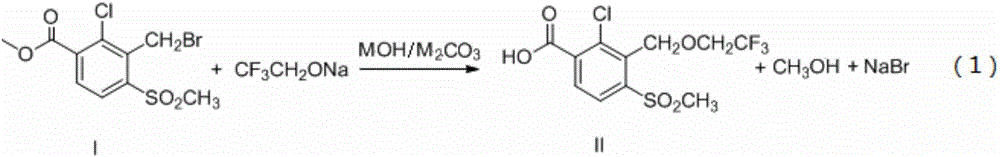
[0025] Step a: get 1.1mol (134.2g) sodium trifluoroethoxide, 1.2mol (48g) sodium hydroxide, 1.0mol (318g) 2-chloro-3-bromomethyl-4-methylsulfonylbenzoate (compound 1), 1000mL of N,N-dimethylformamide was added to the reactor, and after 5 hours of heat preservation at 30°C, after the N,N-dimethylformamide in the reaction liquid was evaporated under reduced pressure, the raffinate was Add 300mL of water, adjust the pH to weak acidity with 35% hydrochloric acid, cool and crystallize, filter, wash with water, and dry to obtain compound II, weighing 314.8g, yield 91%, wherein, N,N-Dimethylformamide was used as Reaction solvent can also be used in hexane, cyclohexane, benzene, toluene, xylene, methylene chloride, 1,2-dichloroethane, chloroform, 1,4-dioxane, ether, tetrahydrofuran, acetonitrile one or more alternatives.
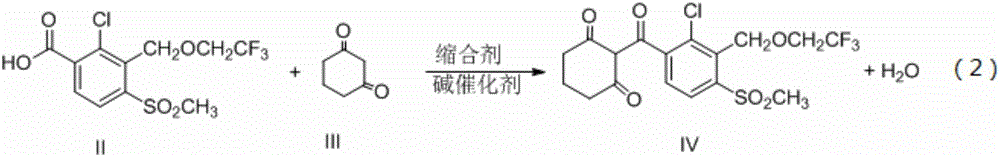
[0026] Step b: Take 1 mol of compound II ob...
Embodiment 2
[0028] Another preparation method of tembotrione provided in this embodiment comprises the following steps:
[0029] Step a: get 1.2mol (146.4g) sodium trifluoroethoxide, 1.2mol (67.2g) potassium hydroxide, 1.0mol (318g) 2-chloro-3-bromomethyl-4-methylsulfobenzoic acid methyl ester ( Compound I), 1000mL N,N-dimethylformamide was added to the reactor, and then reacted at 0°C for 10 hours, and the N,N-dimethylformamide in the reaction solution was distilled off under reduced pressure, and then The residual liquid was added to 300 mL of water, and the pH was adjusted to weak acidity with 35% hydrochloric acid, cooled to crystallize, filtered, washed with water, and dried to obtain Compound II, which was weighed to obtain 321.7 g, with a yield of 93%.
[0030] Step b: Take 1 mol of compound II obtained from the above reaction, dissolve it in 1000 mL of dichloromethane, and then add 1.3 mol (145.6 g) of 1,3-cyclohexanedione at a temperature of 8-15°C within half an hour (Compound ...
Embodiment 3
[0032] Another preparation method of tembotrione provided in this embodiment comprises the following steps:
[0033] Step a: get 1.1mol (134.2g) sodium trifluoroethoxide, 1.2mol (129.6g) sodium carbonate, 1.0mol (318g) 2-chloro-3-bromomethyl-4-methylsulfobenzoate (compound 1), 1000mL of N,N-dimethylformamide was added to the reactor, and after 2 hours of heat preservation at 50°C, after the N,N-dimethylformamide in the reaction liquid was evaporated under reduced pressure, the raffinate was Add 300mL of water, adjust the pH to weak acidity with 35% hydrochloric acid, cool and crystallize, filter, wash with water, and dry to obtain compound II, which weighs 311.4g, yield 90%.
[0034] Step b: Take 1 mol of the compound II obtained from the above reaction, dissolve it in 1000 mL of dichloroethane, and then add 1.5 mol (147 g) of 1,3-cyclohexanedione at a temperature of 15-20 ° C within half an hour (Compound III) and 3mol (405g) condensing agent HOBT and 3mol (222g) calcium hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com