Preparation method of Stanolone
A technology of androsandrolone and compound, which is applied in the field of preparation of androsandrolone, can solve the problems of complex process route, easy to pollute the environment, produce more waste water, etc., and achieve the effect of simple process route, high purity and less pollutants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
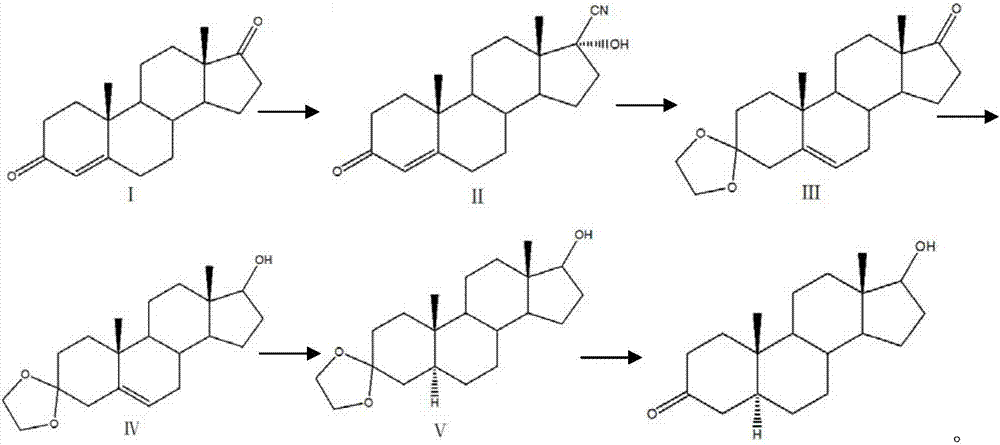
[0023] In the embodiment of the present invention, a kind of preparation method of androsandrolone, the structural formula of described androsandrolone is as follows:
[0024] Its specific preparation method is as follows:
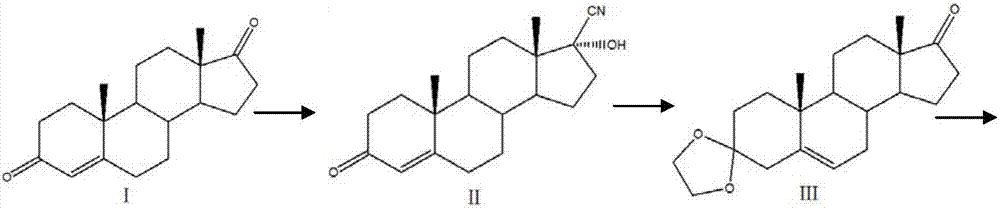
[0025] (1) Compound I reacts with acetone cyanohydrin or sodium cyanide to form compound II with a cyano group. When compound I reacts with acetone cyanohydrin or sodium cyanide, it needs to be used under alkaline conditions. Potassium carbonate, sodium carbonate, triethylamine or sodium hydroxide to adjust pH = 8, temperature 30 degrees Celsius;
[0026] (2) Adjust the pH of the solution of Compound II to be acidic, use PTS, boron trifluoride or acetic acid solution to adjust the pH, and then use ethylene glycol and triethyl orthoformate to form a ketal; then adjust the solution to a strong alkaline Under the conditions, pH ≥ 13, let the cyano group drop, and finally generate compound III;
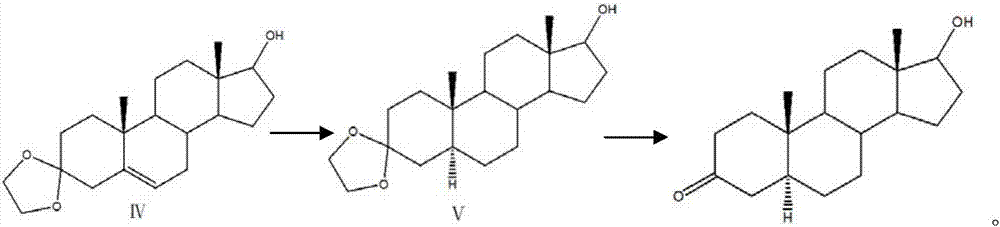
[0027] (3) Add sodium borohydride or potassium borohydride t...
Embodiment 2
[0033] In the embodiment of the present invention, a kind of preparation method of androsandrolone, the structural formula of described androsandrolone is as follows:
[0034] Its specific preparation method is as follows:
[0035] (1) Compound I reacts with acetone cyanohydrin or sodium cyanide to form compound II with a cyano group. When compound I reacts with acetone cyanohydrin or sodium cyanide, it needs to be used under alkaline conditions. Potassium carbonate, sodium carbonate, triethylamine or sodium hydroxide to adjust pH=9, temperature 35 degrees Celsius;
[0036] (2) Adjust the pH of the solution of Compound II to be acidic, use PTS, boron trifluoride or acetic acid solution to adjust the pH, and then use ethylene glycol and triethyl orthoformate to form a ketal; then adjust the solution to a strong alkaline Under the conditions, pH ≥ 13, let the cyano group drop, and finally generate compound III;
[0037] (3) Add sodium borohydride or potassium borohydride to ...
Embodiment 3
[0042] In the embodiment of the present invention, a kind of preparation method of androsandrolone, the structural formula of described androsandrolone is as follows:
[0043] Its specific preparation method is as follows:
[0044] (1) Compound I reacts with acetone cyanohydrin or sodium cyanide to form compound II with a cyano group. When compound I reacts with acetone cyanohydrin or sodium cyanide, it needs to be used under alkaline conditions. Potassium carbonate, sodium carbonate, triethylamine or sodium hydroxide to adjust pH=10, temperature 40 degrees Celsius;
[0045] (2) Adjust the pH of the solution of Compound II to be acidic, use PTS, boron trifluoride or acetic acid solution to adjust the pH, and then use ethylene glycol and triethyl orthoformate to form a ketal; then adjust the solution to a strong alkaline Under the conditions, pH ≥ 13, let the cyano group drop, and finally generate compound III;
[0046] (3) Add sodium borohydride or potassium borohydride to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com