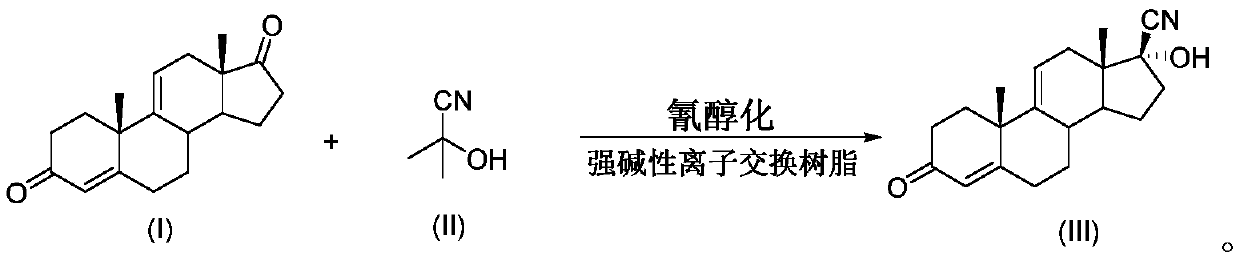
Method for preparing 17beta-cyano-17alpha-hydroxy-9-dehydroandrostenedione
A technology of drostenedione and hydroxyl, which is applied in the field of preparation of 17β-cyano-17α-hydroxy-9-dhydroandrostenedione, which can solve the problem of large amount of three wastes, difficult treatment of cyanide-containing wastewater, low yield, etc. problem, to achieve the effect of continuous production process, easy process chain control, and high mass and heat transfer efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
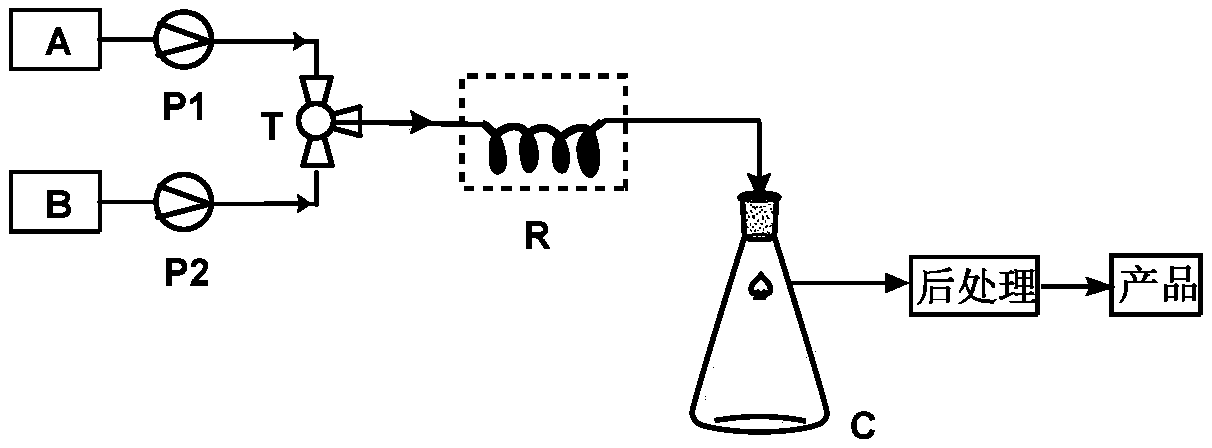
[0024] Such as figure 1 As shown, 9-dehydroandrostenedione (100mmol, 28.4g) was dissolved in 600mL methanol and placed in storage bottle A; acetone cyanohydrin (200mmol, 17g) was dissolved in 600mL methanol and placed in storage bottle B . The materials of storage bottle A and storage bottle B are conveyed by metering pumps respectively, enter the mixer for mixing, and the mixed raw material liquid continuously enters the tubular reactor for cyanohydrinization reaction (the inner diameter of the pipeline is 0.31cm, the length of the pipeline is 5m, and the inner diameter of the pipeline is 5m. Fill with strong basic ion exchange resin, seal both ends with Floxacin, control the molar flow ratio of 9-dehydroandrostenedione: acetone cyanohydrin to 1:2), keep the temperature of the tubular reactor at 25°C, mix The residence time of the raw material liquid in the tubular reactor is 2h. The feed liquid after the reaction enters the receiving bottle C, and after the feed liquid in ...
Embodiment 2
[0027] 9-Dehydroandrostenedione (100mmol, 28.4g) was dissolved in 600mL tetrahydrofuran and placed in storage bottle A; acetone cyanohydrin (200mmol, 17g) was dissolved in 600mL tetrahydrofuran and placed in storage bottle B. The materials of storage bottle A and storage bottle B are conveyed by metering pumps respectively, enter the mixer for mixing, and the mixed raw material liquid continuously enters the tubular reactor for cyanohydrinization reaction (the inner diameter of the pipeline is 0.31cm, the length of the pipeline is 5m, and the inner diameter of the pipeline is 5m. Fill with strong basic ion exchange resin, seal both ends with Floxacin, control the molar flow ratio of 9-dehydroandrostenedione: acetone cyanohydrin to 1:2), keep the temperature of the tubular reactor at 25°C, mix The residence time of the raw material liquid in the tubular reactor is 2h. The feed liquid after the reaction enters the receiving bottle C, and after the feed liquid in the receiving bo...
Embodiment 3
[0029] 9-Dehydroandrostenedione (100mmol, 28.4g) was dissolved in 600mL acetone and placed in storage bottle A; acetone cyanohydrin (200mmol, 17g) was dissolved in 600mL acetone and placed in storage bottle B. The materials of storage bottle A and storage bottle B are conveyed by metering pumps respectively, enter the mixer for mixing, and the mixed raw material liquid continuously enters the tubular reactor for cyanohydrinization reaction (the inner diameter of the pipeline is 0.31cm, the length of the pipeline is 5m, and the inner diameter of the pipeline is 5m. Fill with strong basic ion exchange resin, seal both ends with Floxacin, control the molar flow ratio of 9-dehydroandrostenedione: acetone cyanohydrin to 1:2), keep the temperature of the tubular reactor at 25°C, mix The residence time of the raw material liquid in the tubular reactor is 2h. The feed liquid after the reaction enters the receiving bottle C, and after the feed liquid in the receiving bottle is received...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com