A method for pipeline synthesis of key intermediates of Shakubiqu
A technology of sacubitol and chemical synthesis, applied in the direction of organic chemistry and the like, can solve the problems of hidden danger of explosion of raw material azodicarboxylate, reaction of a large number of refrigerants, low yield, etc., to eliminate hidden danger of explosion, simple production process, The effect of large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
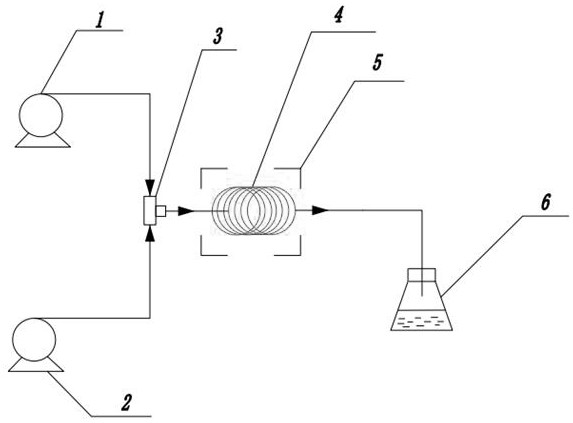
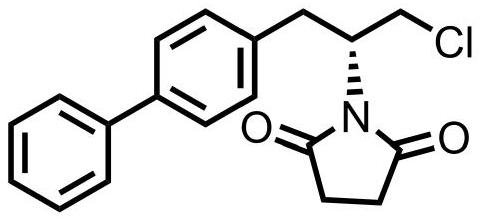
[0028] Example 1. A pipeline synthesis of (R)-1-(1-([1,1'-biphenyl]-4-yl)-3-chloroprop-2-yl)pyrrolidine-2,5- The diketone method, using figure 1 device shown.
[0029] 1) Accurately weigh 200g (S)-1-([1,1'-biphenyl]-4-yl)-3-chloropropan-2-ol and 212g triphenylphosphine, dissolve in 1000mL tetrahydrofuran to prepare solution A ; Weigh again 144g diethyl azodicarboxylate and 92g succinimide, dissolve with 1000mL tetrahydrofuran to form solution B.
[0030] 2) Turn on the constant temperature tank 5, set the reaction temperature to 0°C, and control the temperature of the pipelined reactor 4 (coiled tube with a volume of 4 to 50 mL) through the constant temperature tank 5 to be 0°C;
[0031] 3) The first constant flow pump 1 sets the delivery flow rate of solution A to 10mL / min, the second constant flow pump 2 sets the delivery flow rate of solution B to 10mL / min, and transports solution A and solution B into the T-type mixer 3 for mixing , the mixed solution continuously enter...
Embodiment 2
[0036] Example 2. A pipeline synthesis of (R)-1-(1-([1,1'-biphenyl]-4-yl)-3-chloroprop-2-yl)pyrrolidine-2,5- The diketone approach:
[0037] Reaction conditions repeat Example 1, but different from Example 1: the reaction temperature is replaced by 15°C, under the different coil volumes (volumes) of the pipelined reactor 4, the reaction yield is as shown in Table 2, and the reaction raw material mole The ratio is: n((S)-1-([1,1'-biphenyl]-4-yl)-3-chloropropan-2-ol):n(succinimide):n(azo Diethyl dicarboxylate): n(triphenylphosphine)=1.0:1.0:1.0:1.0.
[0038] Table 2
[0039]
Embodiment 3
[0040] Example 3, a pipeline synthesis of (R)-1-(1-([1,1'-biphenyl]-4-yl)-3-chloropropan-2-yl)pyrrolidine-2,5- The diketone approach:
[0041]Reaction conditions repeat Example 1, but different from Example 1: the reaction temperature is replaced by 25 ° C, under the different coil volumes (volumes) of the pipeline reactor 4, the reaction yield is as shown in Table 3, and the reaction raw material mole The ratio is: n((S)-1-([1,1'-biphenyl]-4-yl)-3-chloropropan-2-ol):n(succinimide):n(azo Diethyl dicarboxylate): n(triphenylphosphine)=1.0:1.0:1.0:1.0.
[0042] table 3
[0043]
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com