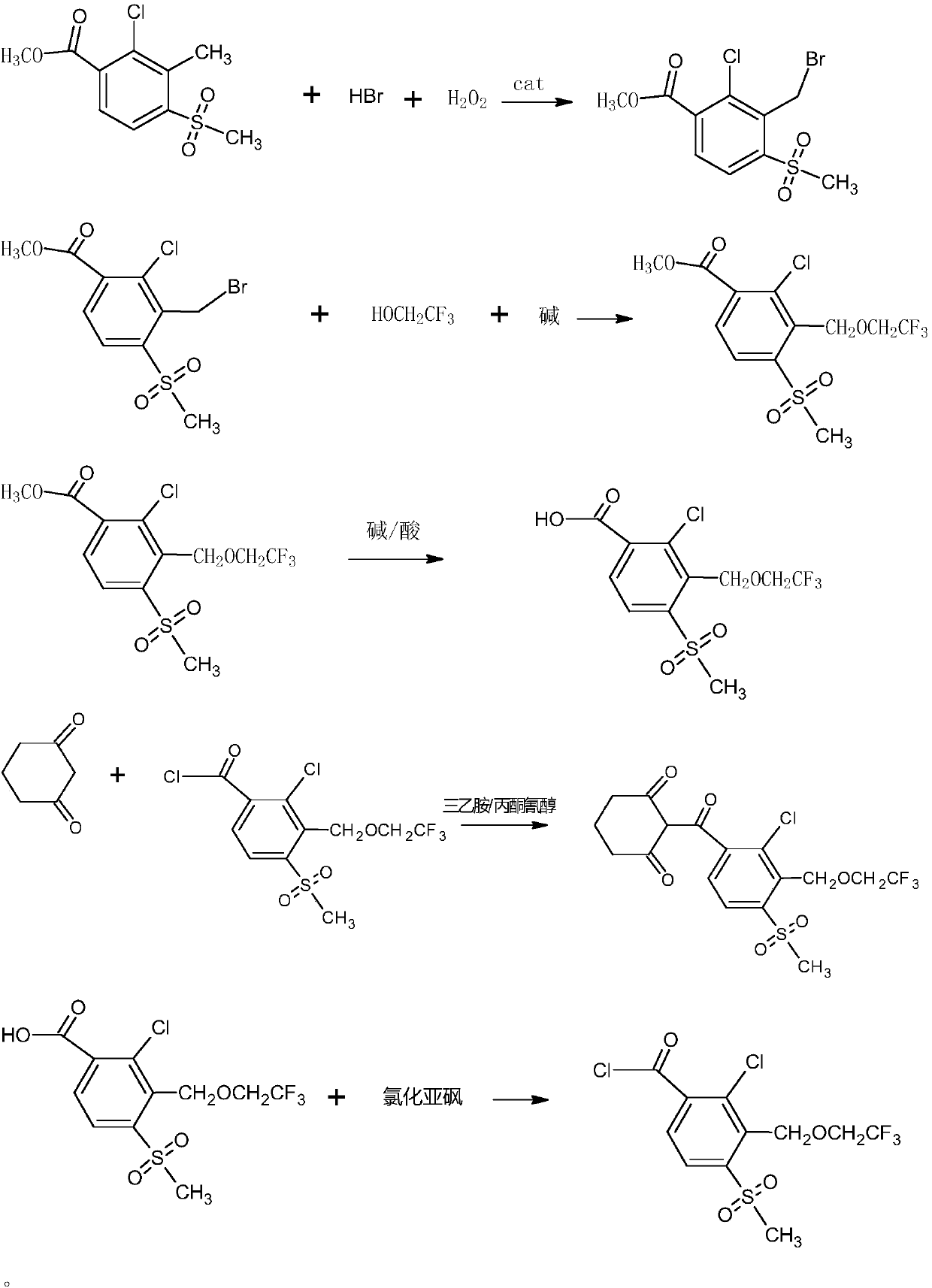
Synthetic process of herbicide tembotrione
A synthesis process, the technology of tembotrione, applied in the field of pesticide technical preparation, can solve problems such as solid waste generation, air and moisture sensitivity, sodium methoxide flammability, etc., to reduce production safety risks, reduce poisoning accidents, Ease of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The present embodiment provides a kind of synthesis technique of tembotrione, comprising the following steps:
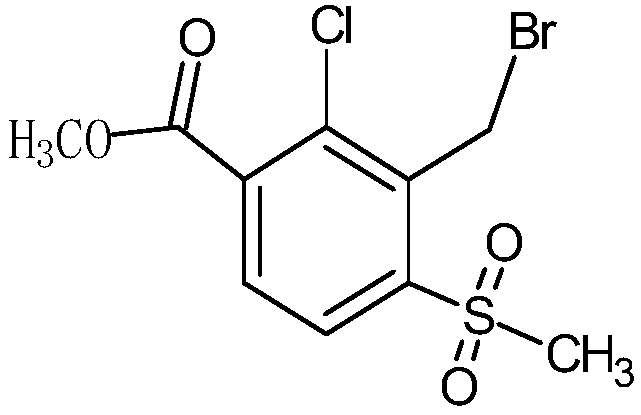
[0038] 1. Synthesis of 2-chloro-3-bromomethyl-4-methylsulfonylbenzoate
[0039]
[0040] 40g 2-chloro-3-methyl-4-methylsulfonylbenzoic acid methyl ester (99.6%, 0.152mol), 200ml dichloroethane, 1g azobisisobutyronitrile (99%, 0.006mol), 46g Add hydrobromic acid (48%, 0.273mol) into a 500ml reaction bottle, add 20.6g of hydrogen peroxide (30%, 0.182mol) dropwise at 75-80°C, continue the reaction after the addition is complete, and track it by LC until the reaction is complete. Layered, the organic layer was washed with water, concentrated, recrystallized with isopropanol, filtered, and dried to obtain 46.67 g of white powder with a purity of 99.0% and a yield of 89%.
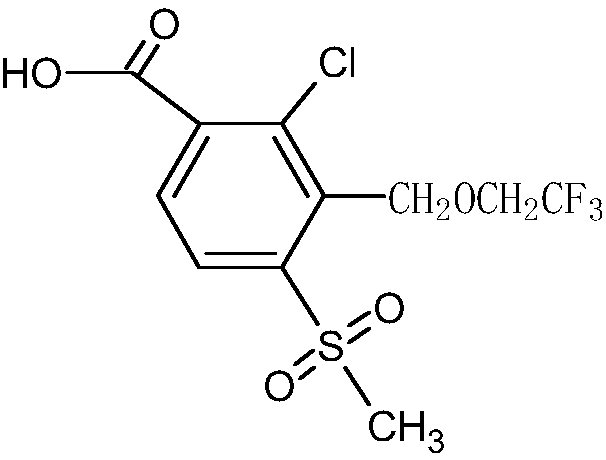
[0041] 2. Synthesis of 2-chloro-3-(2,2,2-trifluoroethoxy)methyl-4-methylsulfonylbenzoic acid
[0042]
[0043] 30g 2-chloro-3-bromomethyl-4-methylsulfonylbenzoic acid methyl ester (99.0%, 0...
Embodiment 2
[0048] The present embodiment provides a kind of synthesis technique of tembotrione, comprising the following steps:
[0049] 1. Synthesis of 2-chloro-3-bromomethyl-4-methylsulfonylbenzoate
[0050]
[0051] 40g 2-chloro-3-methyl-4-methylsulfonylbenzoic acid methyl ester (99.7%, 0.152mol), 200ml dichloromethane, 0.5g azobisisobutyronitrile (99%, 0.003mol), 34g Add hydrobromic acid (48%, 0.202mol) into a 500ml reaction bottle, add 25g of hydrogen peroxide (30%, 0.22mol) dropwise at 45-50°C, continue the reaction after the addition is complete, and track until the reaction is complete by LC. layer, the organic layer was washed with water, concentrated, recrystallized with ethanol, filtered, and dried to obtain 46 g of white powder with a purity of 99.8% and a yield of 88.5%.
[0052] 2. Synthesis of 2-chloro-3-(2,2,2-trifluoroethoxy)methyl-4-methylsulfonylbenzoic acid
[0053]
[0054] 35g 2-chloro-3-bromomethyl-4-methylsulfonylbenzoic acid methyl ester (99.8%, 0.102mol)...
Embodiment 3
[0059] The present embodiment provides a kind of synthesis technique of tembotrione, comprising the following steps:
[0060] 1. Synthesis of 2-chloro-3-bromomethyl-4-methylsulfonylbenzoate
[0061]
[0062] 26.46g 2-chloro-3-methyl-4-methylsulfonylbenzoic acid methyl ester (99.6%, 0.1mol), 200ml dichloroethane, 0.174g m-chloroperoxybenzoic acid (99%, 0.001mol) 16.88g of hydrobromic acid (48%, 0.1mol) was added to a 500ml reaction flask, and 11.33g of hydrogen peroxide (30%, 0.1mol) was added dropwise at 50-55°C. After the addition was completed, the reaction was continued, and LC was followed until the reaction was complete. After the reaction was complete, the layers were separated, the organic layer was washed with water, concentrated, recrystallized with isopropanol, filtered, and dried to obtain 31.33 g of white powder with a purity of 98.0% and a yield of 90%.
[0063] 2. Synthesis of 2-chloro-3-(2,2,2-trifluoroethoxy)methyl-4-methylsulfonylbenzoic acid
[0064] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com