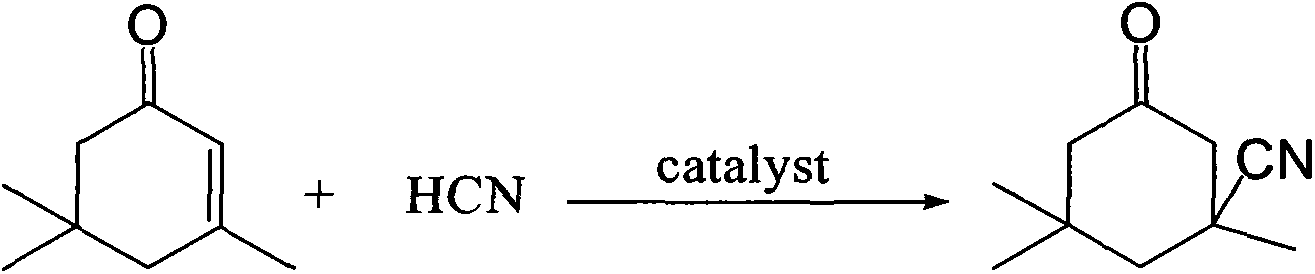
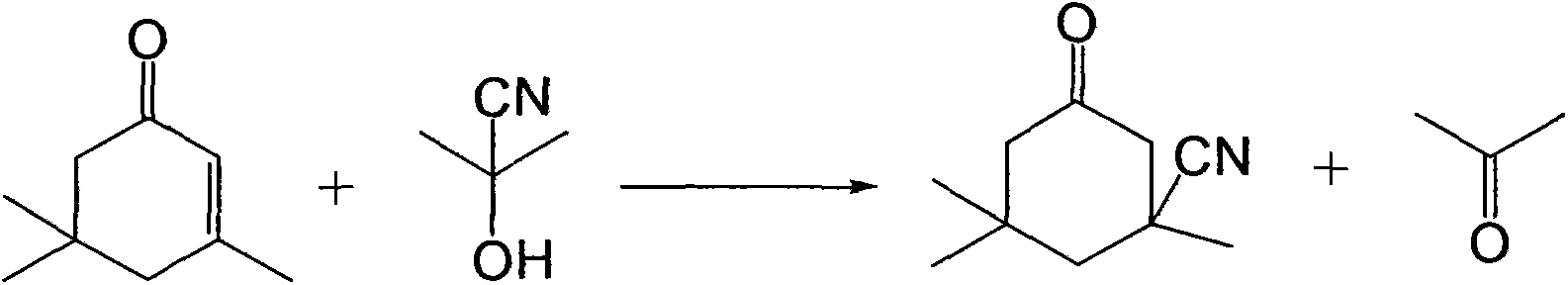
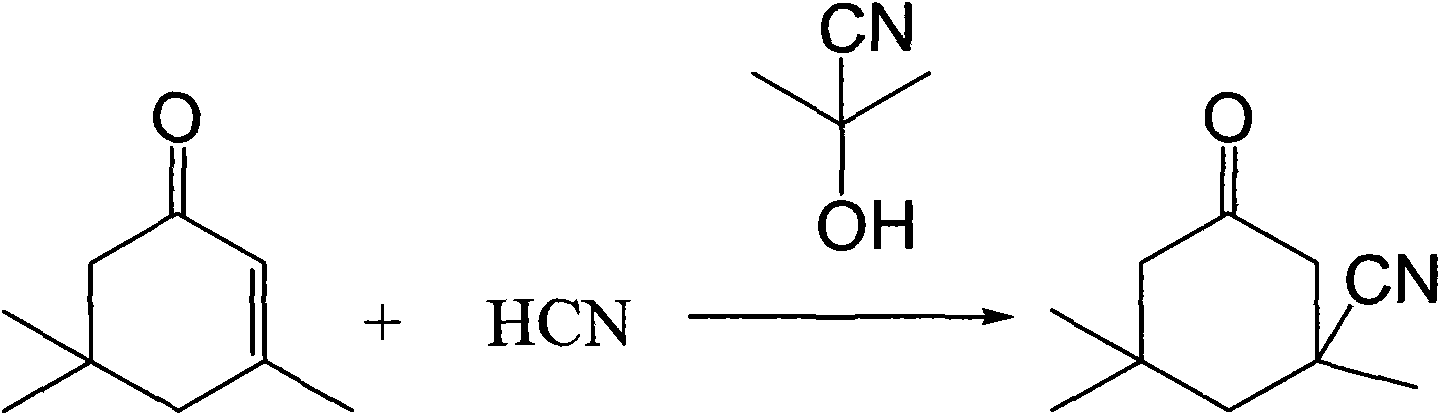
Industrial production method of 3-cyano-3,5,5-trimethylcyclohexanone
A technology of isophorone and isophoronenitrile, applied in the field of organic chemical synthesis, can solve problems such as difficult handling of solid cyanide-containing waste, high requirements for production equipment, and difficulty in industrialized production, and achieve low production cost and simple process , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 138g (1.0mol) of isophorone to a four-neck flask with stirring and a thermometer, raise the temperature to 100°C, add 4.3g (0.05mol) of acetone cyanohydrin, and feed hydrogen cyanide gas to control the amount of gas At 40g (1.5mol), keep the temperature at 100°C, react for 1 hour, cool down to 5°C, filter, and dry to obtain 153g of light yellow isophoronenitrile product with a content of 97.3% and a yield of 95.6%.
Embodiment 2
[0030] Add 138g (1.0mol) of isophorone to a four-necked flask with stirring and a thermometer, raise the temperature to 200°C, add 0.85g (0.01mol) of acetone cyanohydrin, and feed hydrogen cyanide gas to control the amount of gas At 33g (1.2mol), keep the temperature at 200°C, react for 0.5 hours, cool down to 0°C, filter, and dry to obtain 146.4g of light yellow isophoronenitrile product with a content of 95.3% and a yield of 93.2%.
Embodiment 3
[0032] Add 138g (1.0mol) of isophorone to a four-neck flask with stirring and a thermometer, raise the temperature to 150°C, add 1.7g (0.02mol) of acetone cyanohydrin, and feed hydrogen cyanide gas to control the amount of gas At 35g (1.3mol), keep the temperature at 150°C, react for 0.7 hours, cool down to -5°C, filter, and dry to obtain 152.8g of light yellow isophoronenitrile product with a content of 98.3% and a yield of 94.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com