Benzoyl parazole compound as well as synthesis method thereof and application of same as herbicide
A technology for benzoylpyrazoles and benzoylpyrazoles is applied in the field of benzoylpyrazoles and their synthesis, and can solve the problems of low herbicidal activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
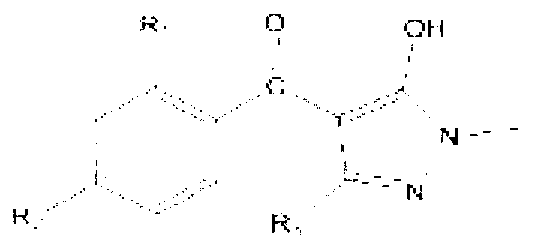
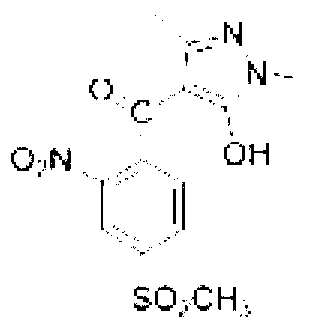
[0032] Specific embodiment one: the synthetic method of present embodiment 1,3-dimethyl-5-hydroxyl-4-(2-nitro-4-thiamphenicol) benzoylpyrazole is realized according to the following steps:
[0033] 1. Add 0.1mol of 1,3-dimethyl-5-pyrazolone, 80mL of acetonitrile and 0.125mol of triethylamine into the four-neck flask and stir evenly. Control the reaction temperature in a water bath to 20°C, and then dropwise add 40ml 0.105mol of 2-nitro-4-thiamphenicol benzoyl chloride dissolved in acetonitrile, stirred and reacted at room temperature for 4h, evaporated the solvent under reduced pressure to obtain a solid product, the solid product was dissolved in 50mL of dichloromethane, washed with 100mL of water, separated The solvent was then distilled off under reduced pressure to obtain 2-nitro-4-thiamphenicol-[1,3-dimethyl-pyrazol-5-yl]-ester;
[0034] 2. Add 150mL of acetonitrile and 0.2ml of acetone cyanohydrin with a purity of 98.5% to the reaction vessel, and then add 0.1mol of 2-ni...
specific Embodiment approach 2
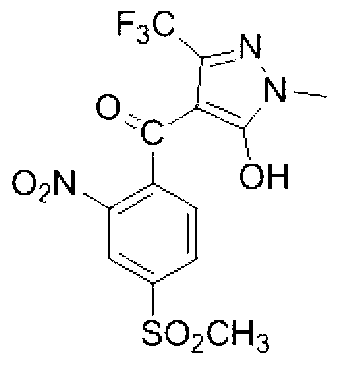
[0041] Specific embodiment two: the synthetic method of present embodiment 1-methyl-3-trifluoromethyl-5-hydroxyl-4-(2-nitro-4-thiamphenicol) benzoylpyrazole is realized according to the following steps :
[0042] 1. Add 0.1mol of 1-methyl-3-trifluoromethyl-5-pyrazolone, 80mL of acetonitrile and 0.125mol of triethylamine into the four-necked flask and stir evenly. Control the reaction temperature in a water bath to 20°C, and then Add dropwise 0.105 mol of 2-nitro-4-thiamphenicol benzoyl chloride dissolved in 40 ml of acetonitrile, stir and react at room temperature for 4 h, evaporate the solvent under reduced pressure to obtain a solid product, and dissolve the solid product in 50 mL of dichloromethane, After washing with 100 mL of water and separating the liquid, the solvent was evaporated under reduced pressure to obtain 2-nitro-4-thiamphenicol-[1-methyl-3-trifluoromethyl-pyrazol-5-yl]-ester;
[0043] 2. Add 150mL of acetonitrile and 0.2ml of acetone cyanohydrin with a purit...
specific Embodiment approach 3
[0050] Specific embodiment three: the synthetic method of present embodiment 1-methyl-3-cyclopropyl-5-hydroxyl-4-(2-nitro-4-thiamphenicol) benzoylpyrazole is realized according to the following steps:
[0051] 1. Add 0.1mol of 1-methyl-3-cyclopropyl-5-pyrazolone, 80mL of acetonitrile and 0.125mol of triethylamine into the four-neck flask and stir evenly. Control the reaction temperature in a water bath to 20°C, then drop Add 0.105 mol of 2-nitro-4-thiamphenicol benzoyl chloride dissolved in 40 ml of acetonitrile, stir and react at room temperature for 4 h, evaporate the solvent under reduced pressure to obtain a solid product, dissolve the solid product in 50 mL of dichloromethane, and dissolve the solid product in 100 mL of After washing with water and liquid separation, the solvent was evaporated under reduced pressure to obtain 2-nitro-4-thiamphenicol-[1-methyl-3-cyclopropyl-pyrazol-5-yl]-ester;
[0052] 2. Add 150mL of acetonitrile and 0.2ml of acetone cyanohydrin with a p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com