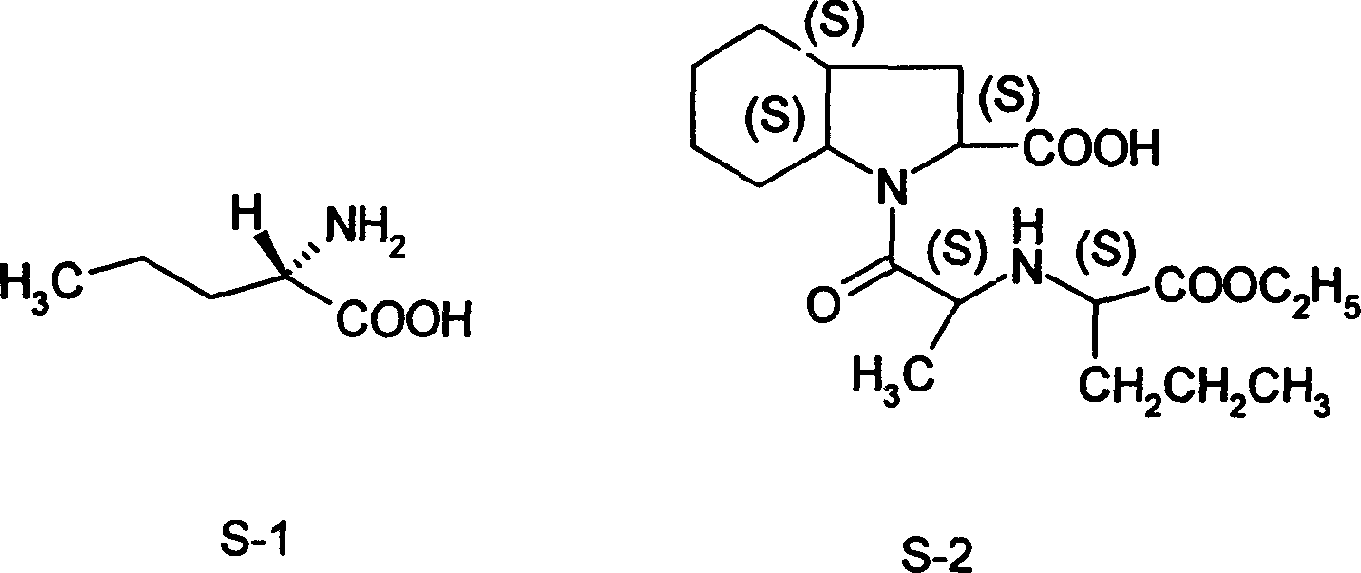
Synthesis method of L-n-valaine
A synthesis method and technology of norvaline, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems that it is difficult to meet the production needs, and the chemical synthesis method has not been used yet, and achieves small investment, The effect of convenient production and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
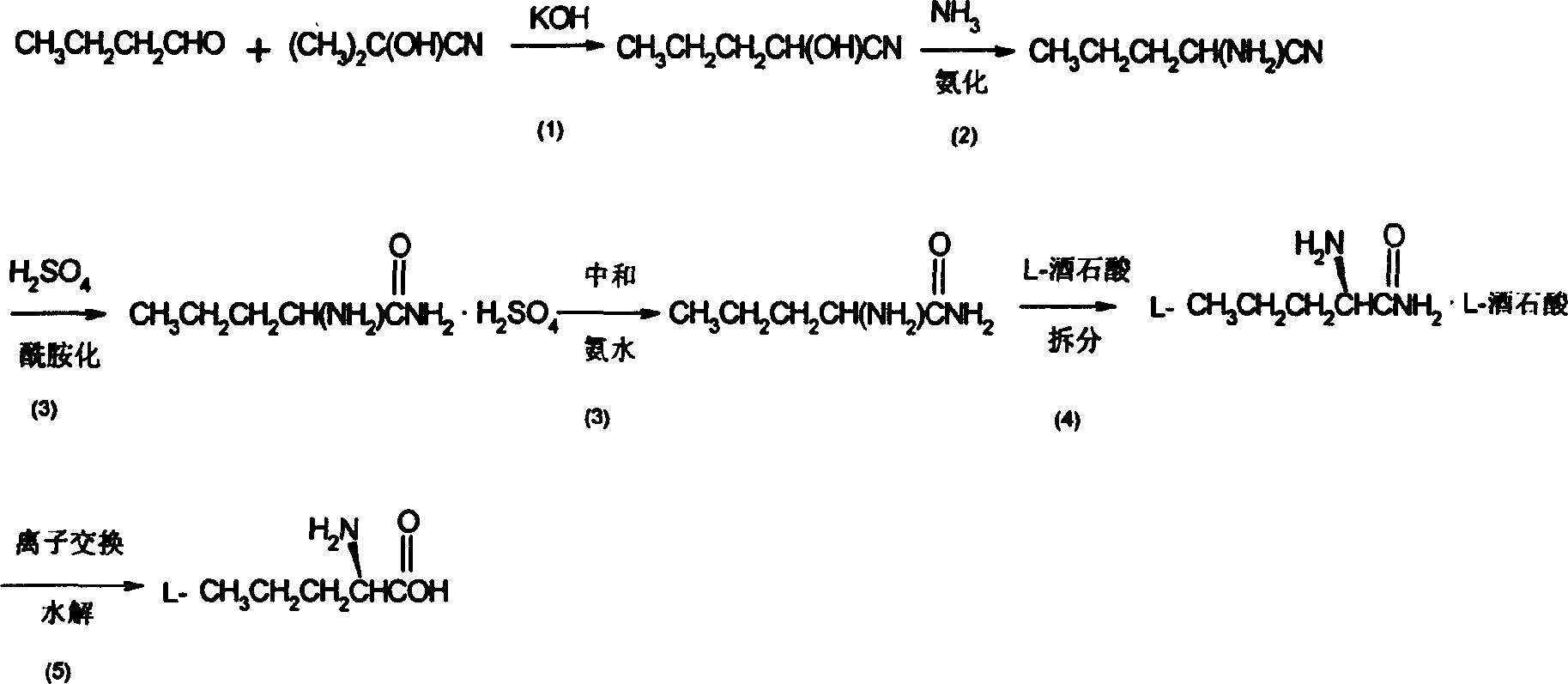
[0019] Embodiment 1: a kind of synthetic method of L-norvaline, take n-butyraldehyde and acetone cyanohydrin as main starting raw material, make through following steps successively:
[0020] (1) cyanation, i.e. the preparation of butyraldehyde cyanohydrin:
[0021] Acetone cyanohydrin (8.5 g, 0.1 mol) was cooled to 0°C, and 10% methanolic potassium hydroxide solution was added dropwise with stirring until the pH value was about 8 and stopped. Control the temperature at 5-10°C, add n-butyraldehyde (7.92g, 0.11mol) dropwise within 20min, keep the temperature and react for 15h after the dropwise addition is complete (take the reaction solution at this time for GC analysis, the content of acetone cyanohydrin <1% , n-butyraldehyde content<3%); after the reaction, add phosphoric acid for neutralization, and adjust the pH value to 3-3.3. The reaction solution was filtered, and the filtrate was concentrated and used directly for the next step of synthesis.
[0022] (2) ammoniation,...
Embodiment 2
[0032] Embodiment 2: a kind of synthetic method of L-norvaline, take n-butyraldehyde and acetone cyanohydrin as main starting raw material, make through following steps successively:
[0033] (1) cyanation, i.e. the preparation of butyraldehyde cyanohydrin:
[0034] Acetone cyanohydrin (8.5 g, 0.1 mol) was cooled to 0° C., and 10% methanolic potassium hydroxide solution was added dropwise with stirring until the pH value was about 7 and stopped. Control the temperature at 10-15°C, add n-butyraldehyde (14g, 0.19mol) dropwise within 20min, keep the temperature for 10h and stop after the dropwise addition; after the reaction, add phosphoric acid for neutralization and adjust the pH value to 3-3.3. The reaction solution was filtered, and the filtrate was concentrated and used directly for the next step of synthesis.
[0035] (2) ammoniation, i.e. the preparation of aminovaleronitrile:
[0036] Add liquid ammonia (11.9 g, 0.7 mol) into the autoclave, press butyraldehyde cyanohydr...
Embodiment 3
[0045] Embodiment 3: a kind of synthetic method of L-norvaline, take n-butyraldehyde and acetone cyanohydrin as main starting raw material, make through following steps successively:
[0046] (1) cyanation, i.e. the preparation of butyraldehyde cyanohydrin:
[0047] Acetone cyanohydrin (8.5 g, 0.1 mol) was cooled to 0° C., and 10% methanolic sodium hydroxide solution was added dropwise with stirring until the pH value was about 9 and stopped. Control the temperature at 0-5°C, add n-butyraldehyde (10.8g, 0.15mol) dropwise within 20min, keep the temperature and react for 5h after the dropwise addition is completed; add phosphoric acid to neutralize after the reaction, adjust the pH value to 3-3.3 . The reaction solution was filtered, and the filtrate was concentrated and used directly for the next step of synthesis.
[0048] (2) ammoniation, i.e. the preparation of aminovaleronitrile:
[0049] Add liquid ammonia (15.3 g, 0.9 mol) into the autoclave, press butyraldehyde cyanoh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com