Preparation method of alpha-hydroxypyridine by acetone cyanohydrin
A technology of acetone cyanohydrin and hydroxynitrile, which is applied in the field of preparation of α-hydroxynitrile, can solve the problems of complex preparation method, high catalyst toxicity, long reaction time, etc., and achieve the advantages of avoiding side reactions, simplifying post-treatment process, and shortening reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
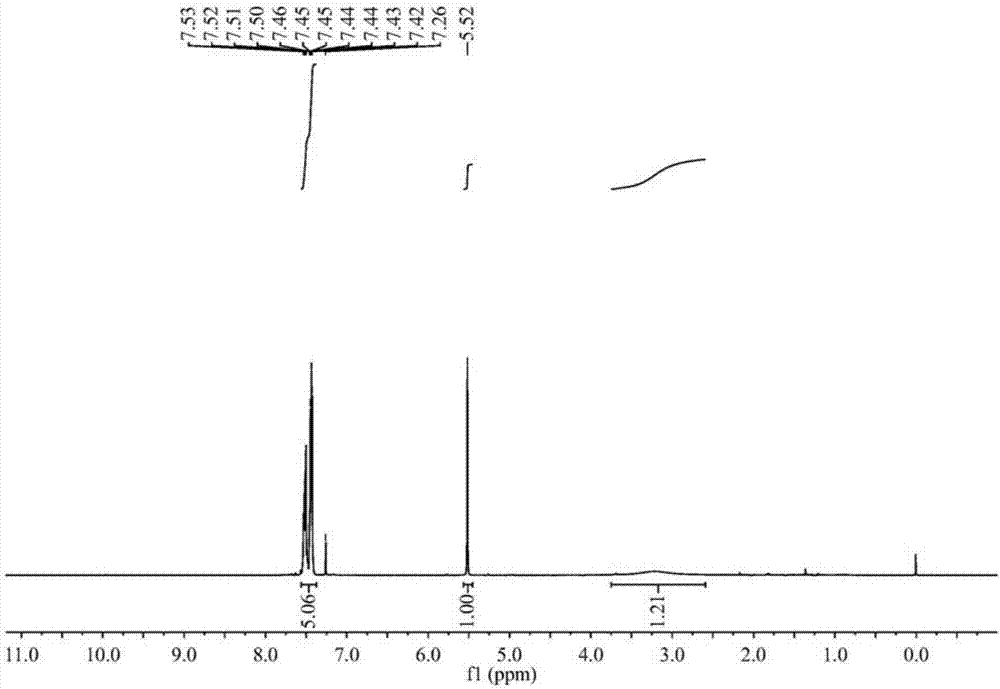
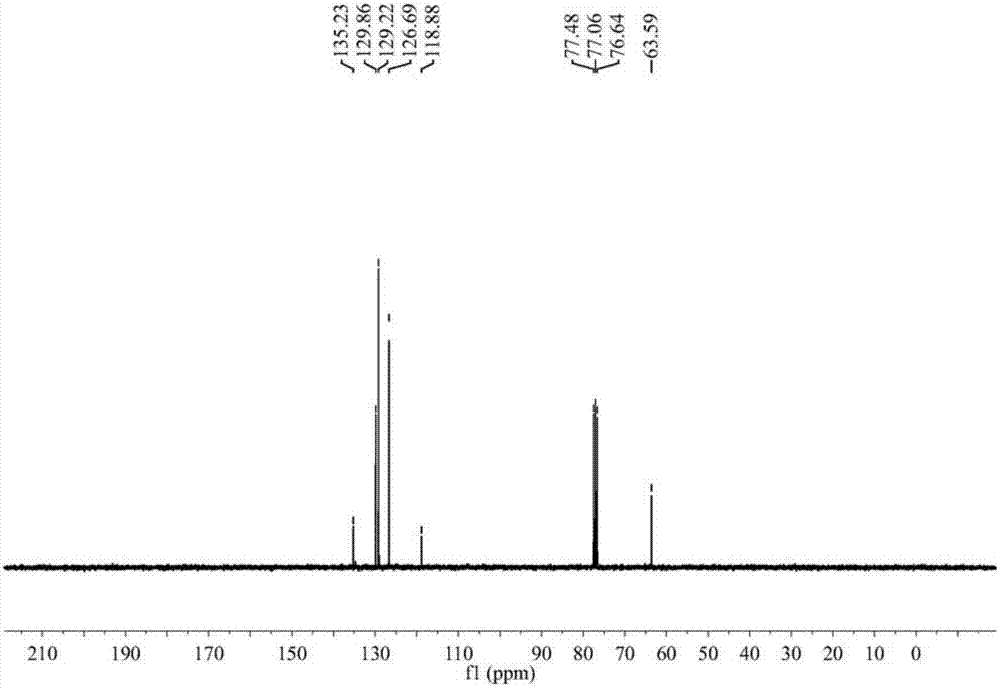
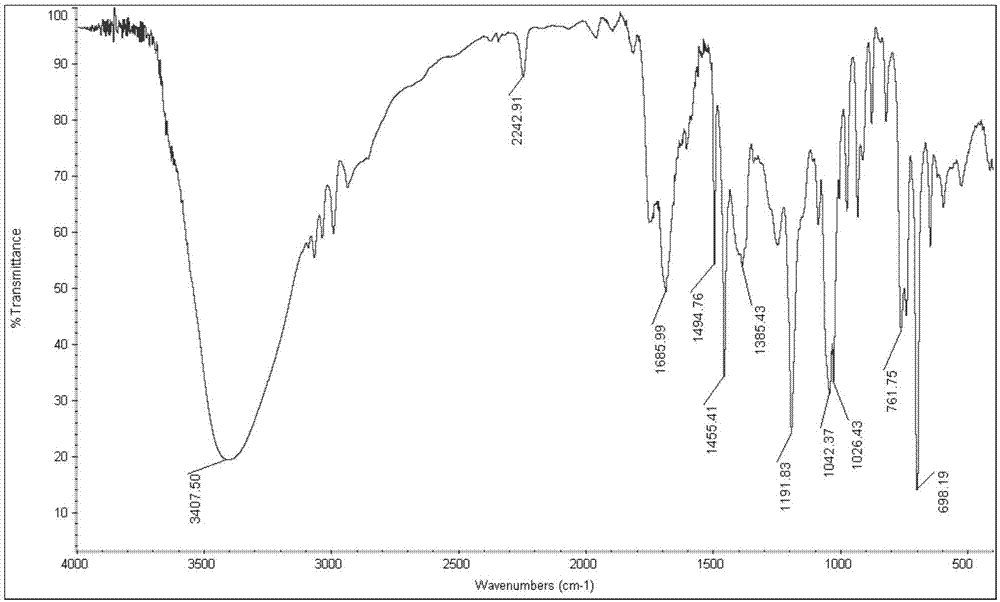
[0025] Specific embodiment one: the present embodiment utilizes acetone cyanohydrin to prepare the method for α-hydroxynitrile, comprises the following steps:
[0026] One, the preparation of catalyst:
[0027] immersing the cation exchange resin in a 20% by weight organic amine methanol solution for 6-8 hours, filtering, washing with methanol for 3-5 times, and drying to obtain a catalyst;
[0028] Two, the preparation of α-hydroxynitrile:
[0029] Dissolve acetone cyanohydrin and aromatic aldehyde in methanol at a molar ratio (1.1 to 1.3): 1, add a catalyst, react at room temperature for 2 to 10 hours, and obtain α-hydroxynitrile; wherein the mass of the catalyst is 3% to 10% of the mass of the aromatic aldehyde ;
[0030] The catalyst was filtered off, methanol and by-product acetone were distilled off under reduced pressure, the residue was extracted and separated by adding ethyl acetate and water, the ethyl acetate layer was washed 2 to 3 times with 20% brine, and after...
specific Embodiment approach 2
[0031] Embodiment 2: This embodiment differs from Embodiment 1 in that the cation exchange resin in step 1 is a macroporous weakly acidic acrylic cation exchange resin. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0032] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that the organic amine in step 1 is 2,5-lutidine, 2-picoline, 2-aminopyridine, 4-aminopyridine, Pyrazine, piperidine, triallylamine, tributylamine or dimethyloctylamine. Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com