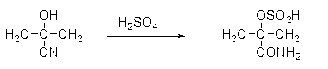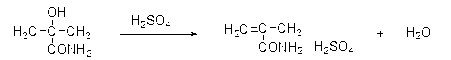Preparation and purification method of clean methacrylamide
A technology of methacrylamide and purification method, which is applied in the field of synthesis of methacrylamide, can solve the problems of waste water environmental pollution, product loss, etc., and achieve the effects of avoiding pollution, increasing yield, and reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
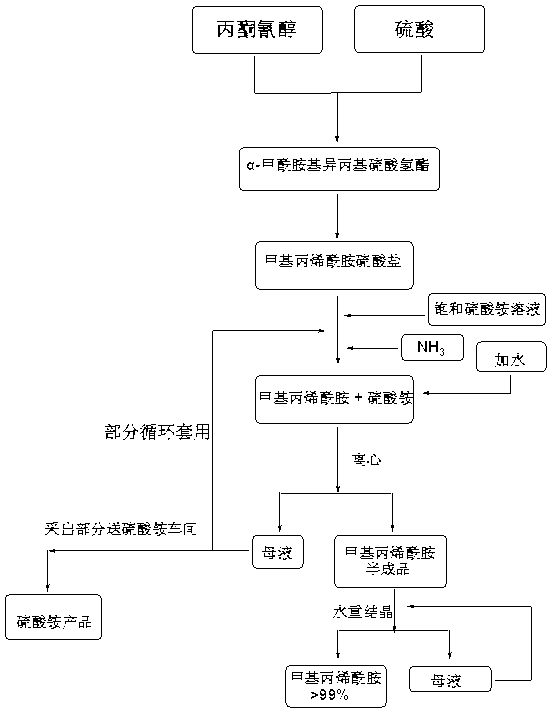
[0029] 1) Put concentrated sulfuric acid (1360 kg) and acetone cyanohydrin (850 kg) into the reactor at a ratio of 1.6:1 (mass ratio) through a rotameter, and hydrolyze the acetone cyanohydrin with concentrated sulfuric acid to form α-formamide Because the reaction is exothermic at this time, the cooling system is turned on to keep the reaction temperature in the pot at 90°C, and the product overflows from the overflow of the reactor and enters the tubular reactor;
[0030] 2) The above-mentioned reactants continue to chemically react with concentrated sulfuric acid in the tubular reactor. Under the action of concentrated sulfuric acid, α-formamidoisopropyl hydrogensulfate is dehydrated to form methacrylamide sulfate. This reaction is endothermic The reaction is heated to 130°C with steam. Because the temperature is too low, the chemical reaction is incomplete, and the temperature is too high, which is easy to cause polymerization or carbonization, so temperature control is a key ...
Embodiment 2
[0034] Example 2 (Mother liquor circulation is applied 1)
[0035] 1) Put concentrated sulfuric acid (1360 kg) and acetone cyanohydrin (850 kg) into the reactor at a ratio of 1.6:1 (mass ratio) through a rotameter, and hydrolyze the acetone cyanohydrin with concentrated sulfuric acid to form α-formamide Because the reaction is exothermic at this time, the cooling system is turned on to keep the reaction temperature in the pot at 95°C, and the product overflows from the overflow of the reactor and enters the tubular reactor;
[0036] 2) The above-mentioned reactants continue to chemically react with concentrated sulfuric acid in the tubular reactor. Under the action of concentrated sulfuric acid, α-formamidoisopropyl hydrogensulfate is dehydrated to form methacrylamide sulfate. This reaction is endothermic The reaction is heated to 135°C with steam. Because the temperature is too low, the chemical reaction is incomplete, and the temperature is too high, which is easy to cause polym...
Embodiment 3
[0040] Example 3 (Mother liquor circulation application 2)
[0041] 1) Put concentrated sulfuric acid (1360 kg) and acetone cyanohydrin (850 kg) into the reactor at a ratio of 1.6:1 (mass ratio) through a rotameter, and hydrolyze the acetone cyanohydrin with concentrated sulfuric acid to form α-formamide Because the reaction is exothermic at this time, the cooling system is turned on to keep the reaction temperature in the pot at 90°C, and the product overflows from the overflow of the reactor and enters the tubular reactor;
[0042] 2) The above-mentioned reactants continue to chemically react with concentrated sulfuric acid in the tubular reactor. Under the action of concentrated sulfuric acid, α-formamidoisopropyl hydrogensulfate is dehydrated to form methacrylamide sulfate. This reaction is endothermic The reaction is heated to 130°C with steam. Because the temperature is too low, the chemical reaction is incomplete, and the temperature is too high, which is easy to cause poly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com