Eplerenone dispersible tablet
A technology of eplerenone and dispersible tablets, applied in the field of medicine, can solve the problems of poor stability of liquid preparations, low bioavailability, slow disintegration of solid preparations, etc., and achieves reduction of adverse reactions, avoidance of high drug concentration, and fast absorption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Prescription 1: (prescription quantity is 1000 tablets)
[0041]
[0042] 2. Preparation process:
[0043] 1) Pass the active ingredient eplerenone through a 200-mesh sieve;
[0044] 2) Weigh the prescribed amount of eplerenone, and add the prescribed amount of lactose, microcrystalline cellulose, croscarmellose sodium, low-substituted hydroxypropyl cellulose and lauryl Sodium sulfate, mixed evenly; add purified water to moisten the soft material, and granulate with a 24 mesh sieve;
[0045] 3) Wet granules are air-dried at 50-70°C, and sieved with 20 meshes;
[0046] 4) add the lubricant talcum powder and magnesium stearate of prescription quantity, mix homogeneously;
[0047] 5) Tablets.
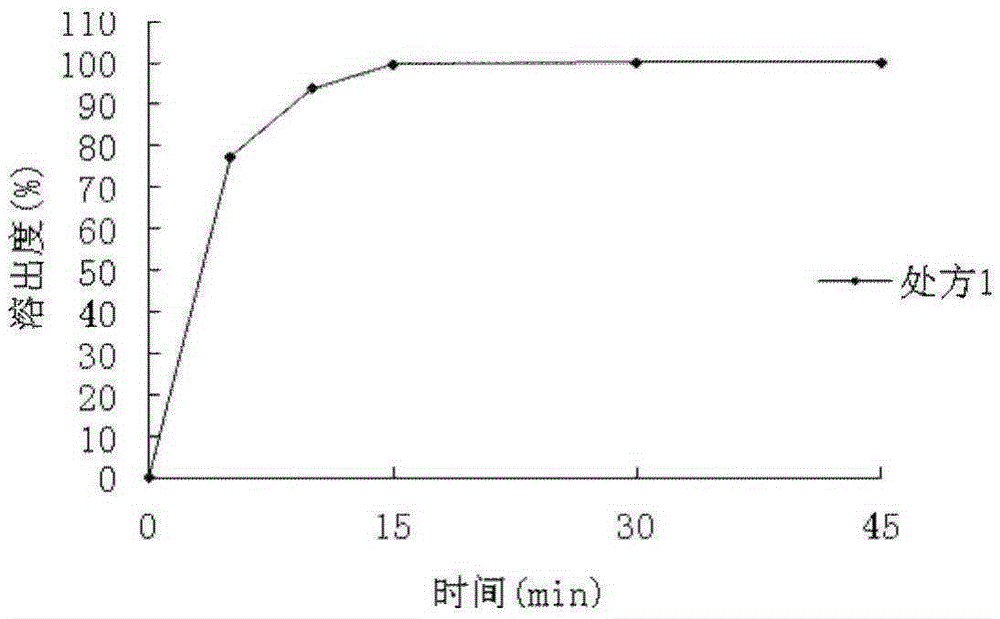
[0048] The dispersion time limit of the dispersible tablet prepared in this embodiment is 48 seconds, and the dissolution rate is 99.85%.
Embodiment 2
[0050] 1. Prescription 2: (prescription quantity is 1000 tablets)
[0051]
[0052] 2. Preparation process:
[0053] 1) Pass the active ingredient eplerenone through a 200-mesh sieve;
[0054] 2) Weigh the prescribed amount of eplerenone, and add the prescribed amount of lactose, microcrystalline cellulose, croscarmellose sodium, low-substituted hydroxypropyl cellulose and lauryl Sodium sulfate, mixed evenly; add purified water to moisten the soft material, and granulate with a 24 mesh sieve;
[0055] 3) Wet granules are air-dried at 50-70°C, and sieved with 20 meshes;
[0056] 4) add the lubricant talcum powder and magnesium stearate of prescription quantity, mix homogeneously;
[0057] 5) Tablets.
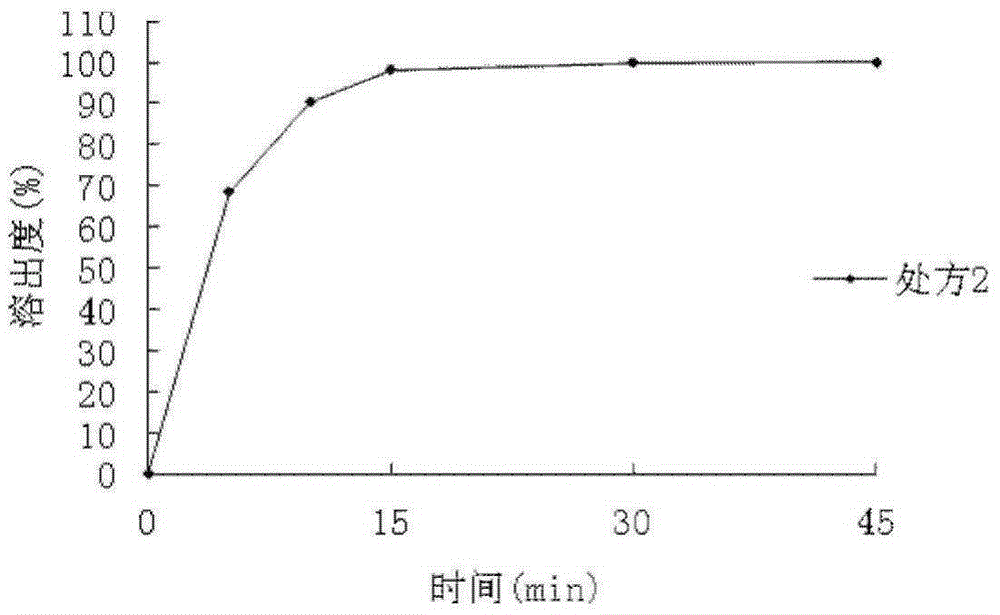
[0058] The dispersion time limit of the dispersible tablets prepared in this example is 1 minute and 19 seconds, and the dissolution rate is 98.34%.
Embodiment 3
[0060] 1. Prescription 3: (prescription quantity is 1000 tablets)
[0061]
[0062]
[0063] 2. Preparation process:
[0064] 1) Pass the active ingredient eplerenone through a 200-mesh sieve;
[0065] 2) Weigh the prescribed amount of eplerenone, and add the prescribed amount of lactose, microcrystalline cellulose, croscarmellose sodium, low-substituted hydroxypropyl cellulose and lauryl Sodium sulfate, mixed evenly; add purified water to moisten the soft material, and granulate with a 24 mesh sieve;
[0066] 3) Wet granules are air-dried at 50-70°C, and sieved with 20 meshes;
[0067] 4) add the lubricant talcum powder and magnesium stearate of prescription quantity, mix homogeneously;
[0068] 5) Tablets.
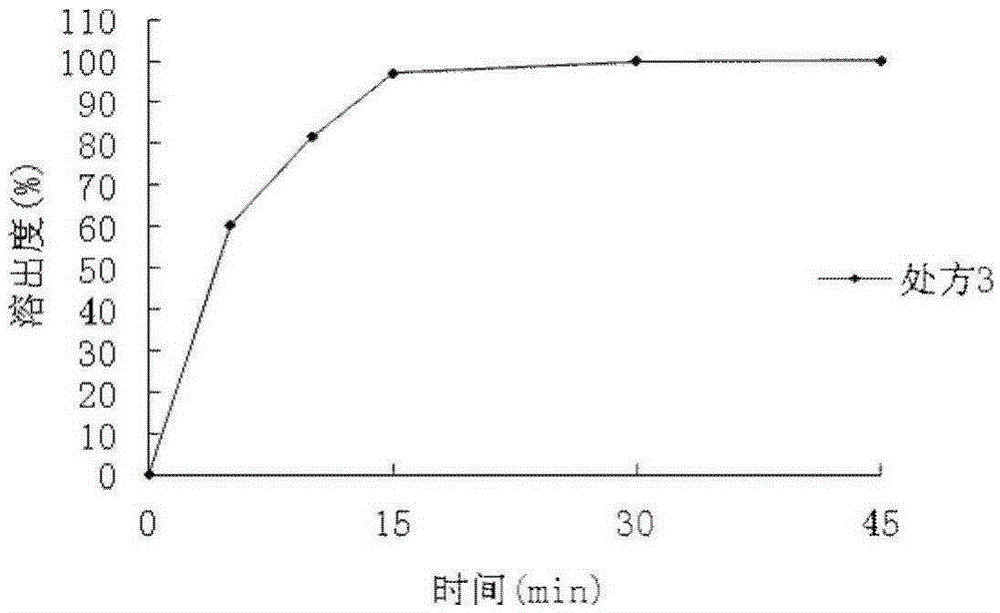
[0069] The dispersion time limit of the dispersible tablets prepared in this example is 1 minute and 54 seconds, and the dissolution rate is 98.88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com