Synthesizing method of eplerenone
A technology of eplerenone and a synthesis method, which is applied in the field of synthesis of steroidal antihypertensive drug eplerenone, can solve the problems of high price and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
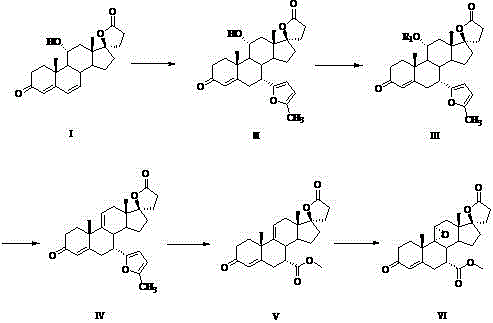
[0018] Implementation Example 1: Synthesis of 7α-(5-methyl-2-furan)-11α-hydroxycanrenone (Ⅱ)
[0019] Add 26.5 g of 11α-hydroxycanrenone to a 500 mL three-neck flask under nitrogen protection, dry 210 mL of nitromethane and 50 mL of dichloromethane. Cool to -22°C, add 12.5g of 2-methylfuran, 45g of anhydrous methanol, and 12g of boron trifluoride etherate in sequence, and keep stirring at -16 to 20°C for 30 hours. Add 52 mL of 15% ammonia water to terminate the reaction. The reaction solution was extracted with dichloromethane, dried over anhydrous sodium sulfate for 4 hours, filtered with suction, and the filtrate was concentrated. The obtained solid was recrystallized with a mixed solvent of ethyl acetate and dichloromethane at a volume ratio of 4:1, filtered, and the filter cake was stored at 40°C Dry in a blast drying oven for 12 hours to obtain a white solid powder with a yield of 75.6%.
Embodiment 2
[0020] Example 2: Synthesis of 7α-(5-methyl-2-furan)-11α-p-methylsulfonylcanrenone (Ⅲ):
[0021] Add 25.0 g of compound II and 125 mL of chloroform into a 500 mL three-necked flask. Cool to 0°C, add 70 mL of anhydrous pyridine, dissolve and add 32.5 g of p-toluenesulfonyl chloride. After 4 hours of reaction, the temperature was naturally raised to 25° C., and the stirring reaction was continued for 22 hours. Add 80 g of water to terminate the reaction, collect the organic phase by liquid separation extraction, dry over anhydrous sodium sulfate for 4 hours, filter, concentrate the filtrate, and recrystallize the solid with a mixed solvent of acetone and ethanol at a volume ratio of 1:2. After filtering, the filter cake was dried in a blast oven at 40°C for 12 hours to obtain a white solid powder with a yield of 90.7%.
Embodiment 3
[0022] Implementation example three: the synthesis of compound IV:
[0023] Add 30.0 g of compound III and 47.0 g of anhydrous sodium acetate into a 500 mL three-necked flask, add 180 mL of anhydrous formic acid, heat to 80° C. for 3 hours, and the reaction is complete. The formic acid was distilled off under reduced pressure, and the residue was added with dichloromethane and water, and separated and extracted. The organic layer was washed with saturated sodium bicarbonate solution, separated, the organic layer was dried with anhydrous sodium sulfate for 4 hours, filtered, the filtrate was concentrated, and the solid was recrystallized with a mixed solvent of ethanol and acetone at a volume ratio of 3:1 to obtain a white filter cake, which was collected rate of 64.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com