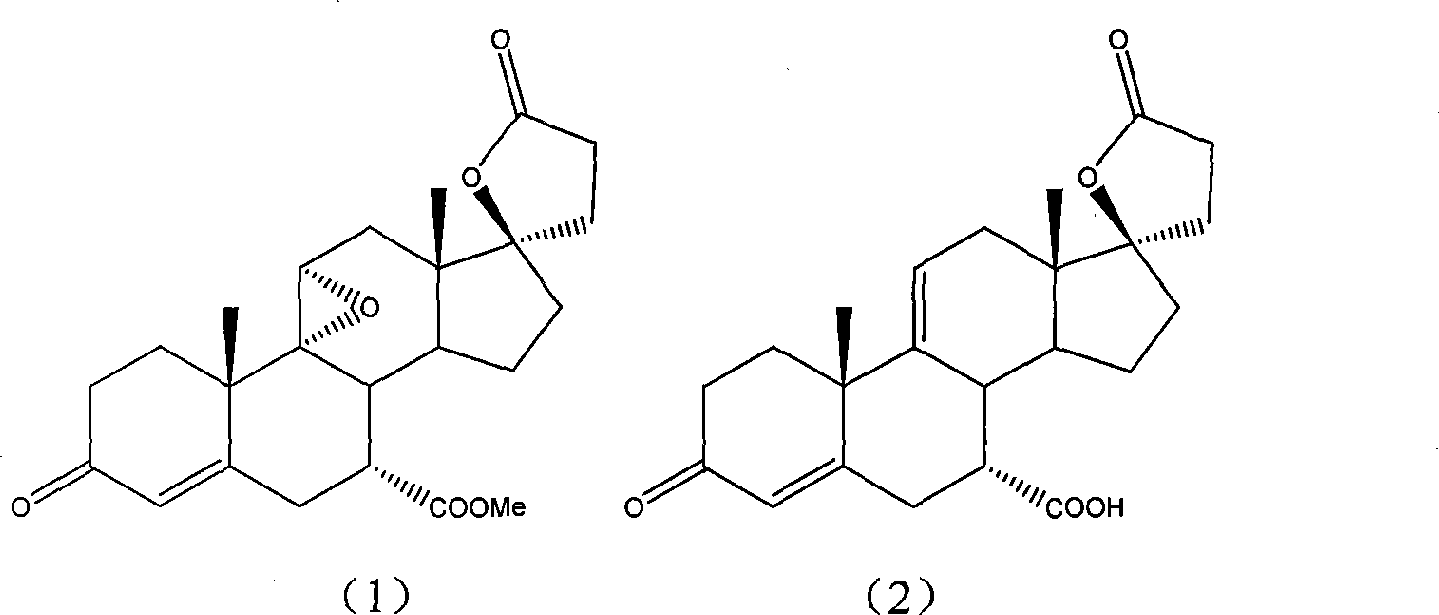
Preparation method of intermediate for preparing eplerenone
A technology for eplerenone and intermediates, which is applied in the field of preparation of intermediates for eplerenone, can solve problems such as being unsuitable for industrial production, and achieve the effects of avoiding product purification difficulties, simple method and improved yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of 17β-hydroxy-7α-vinyl-3-oxo-17α-pregna-4,9(11)-diene-21-carboxylic acid-γ-lactone:
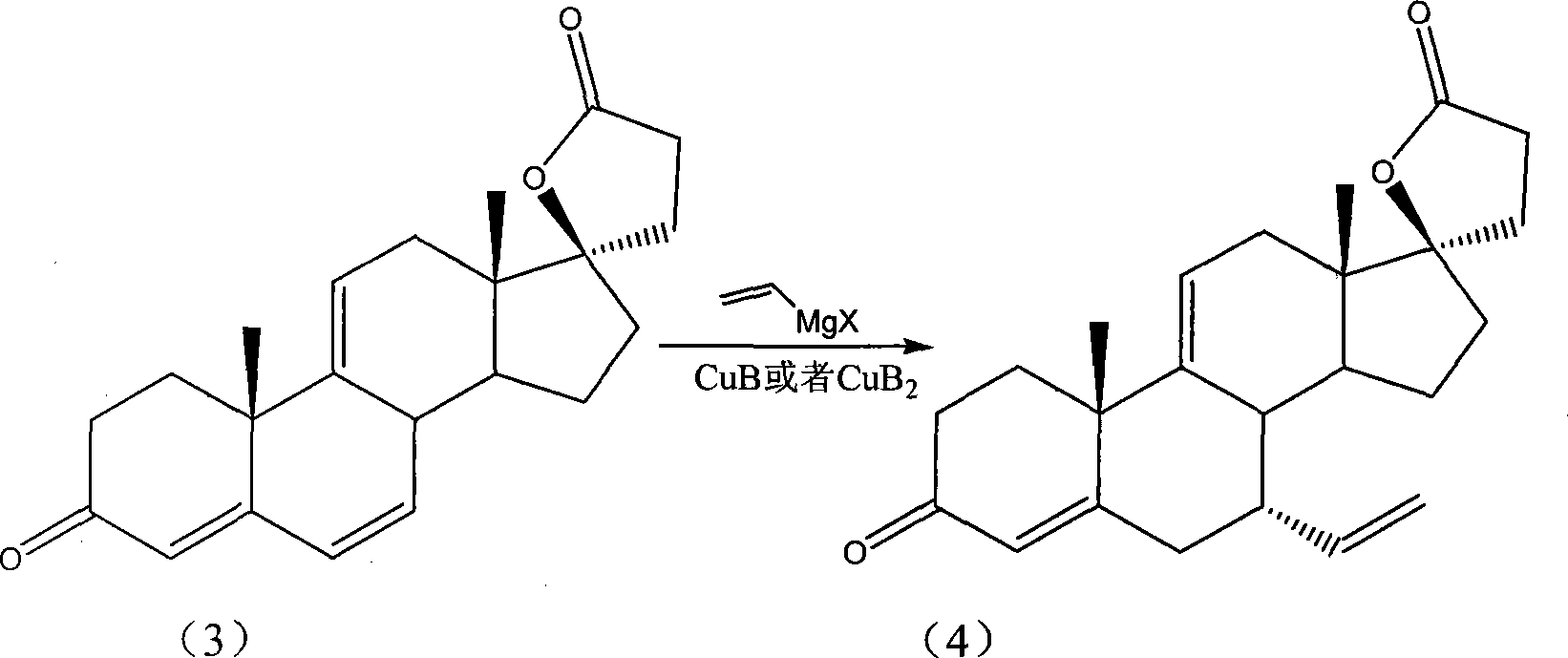
[0032] Add anhydrous tetrahydrofuran 100mL, cuprous chloride (Fw: 99.01, 0.01g, 10mmol) in a dry 250mL three-neck flask equipped with a magnetic stirrer, a thermometer, and a constant pressure dropping funnel in sequence, and lower the temperature to -20°C. The tetrahydrofuran solution (2M, 50mL) of alkenylmagnesium chloride was slowly added dropwise to the system, and the dropwise addition was completed in about 5 minutes. After the dropwise addition was completed, the system was stirred at -20°C for 2 minutes, and then Δ 9(11) Kanrenone (Fw: 338.19, 3.38 g, 10 mmol) was dissolved in 15 mL of anhydrous tetrahydrofuran, and transferred to a constant pressure dropping funnel. Decrease the temperature of the system to -50°C, and the Δ 9(11) - The kanrenone tetrahydrofuran solution was slowly added dropwise to the system, the reaction temperature was controlled at -50°C, an...
Embodiment 2
[0035] Preparation of 17β-hydroxy-7α-vinyl-3-oxo-17α-pregna-4,9(11)-diene-21-carboxylic acid-γ-lactone:
[0036] Add anhydrous diethyl ether 100mL, anhydrous copper acetate (Fw: 181.63, 0.018g, 10mmol) in a dry 250mL three-necked flask equipped with a magnetic stirrer, a thermometer, and a constant pressure dropping funnel in sequence, and maintain the temperature at 20°C. Alkenylmagnesium chloride tetrahydrofuran solution (2M, 60mL) was added to the system all at once. After the dropwise addition was completed, the system was stirred at 20°C for 5 minutes, and then Δ 9(11 )-Kanrenone (Fw: 338.19, 3.38g, 10mmol) was dissolved in 15mL of anhydrous ether, and transferred to a constant pressure dropping funnel. Decrease the temperature of the system to 0°C, and the Δ 9(11) - The ether solution of kanrenone was slowly added dropwise to the system, the reaction temperature was controlled at 0°C, and the dropwise addition was completed in about 5 minutes. After the dropwise addit...
Embodiment 3
[0039] Preparation of 17β-hydroxy-7α-carboxy-3-oxo-17α-pregna-4,9(11)-diene-21-carboxylic acid-γ-lactone:
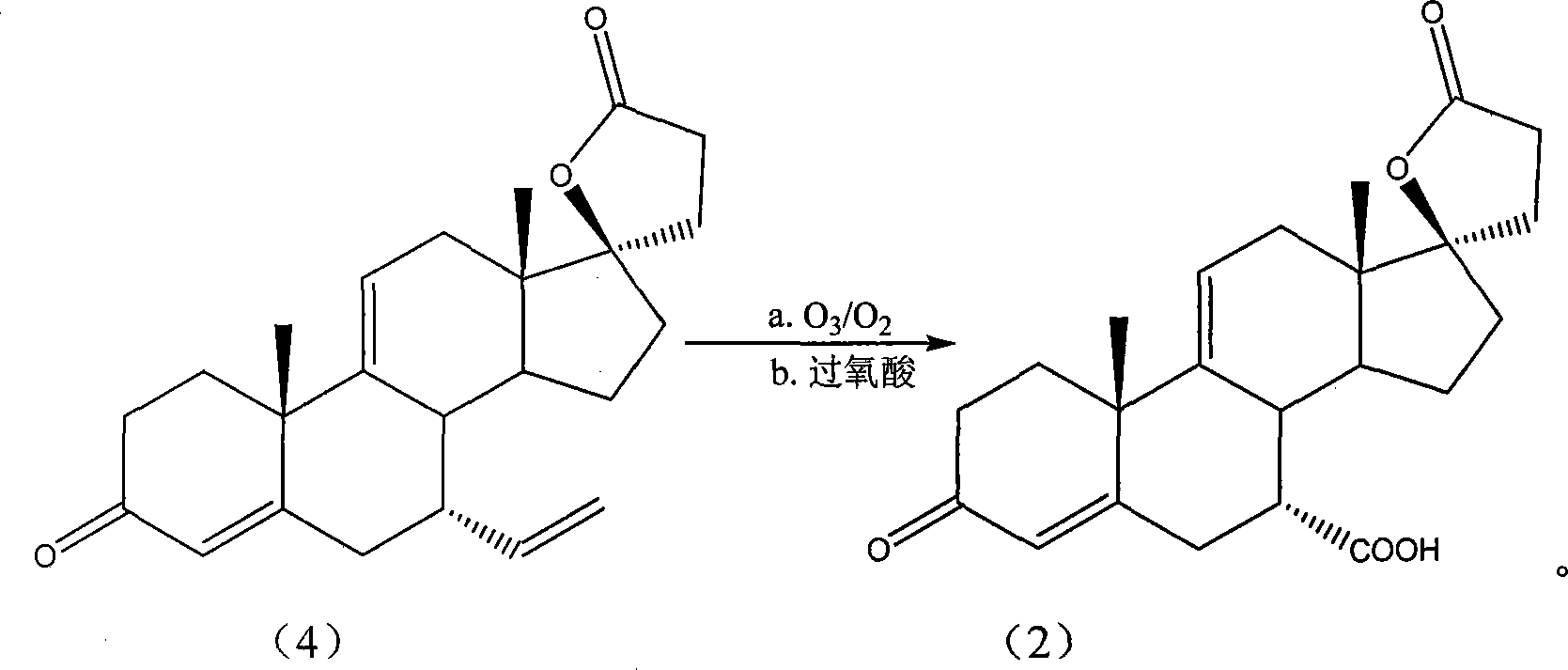
[0040] The 17β-hydroxyl-7α-vinyl-3-oxo-17α-pregna-4,9(11)-diene-21-carboxylic acid-γ-lactone (Fw: 366.49, 10g , 27.3mmol), dissolved in 100mL of dichloromethane, then the system temperature was lowered to -78°C, the mixed gas of ozone and oxygen was slowly introduced into the system, and the reaction temperature was kept at -78°C. After the system turns blue, stop the introduction of ozone. Then the temperature of the system was raised to -10°C, and peracetic acid (Fw: 76.05, 3.11g, 41.0mmol) was diluted in 10mL of dichloromethane, and then slowly added dropwise to the system, the temperature was kept at -3°C, about 30 Minutes dropwise completed. After the dropwise addition was complete, the system was warmed up to room temperature, and stirring was continued for 20 hours. Subsequently, saturated sodium thiosulfate aqueous solution was added dropwise to the system unt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com