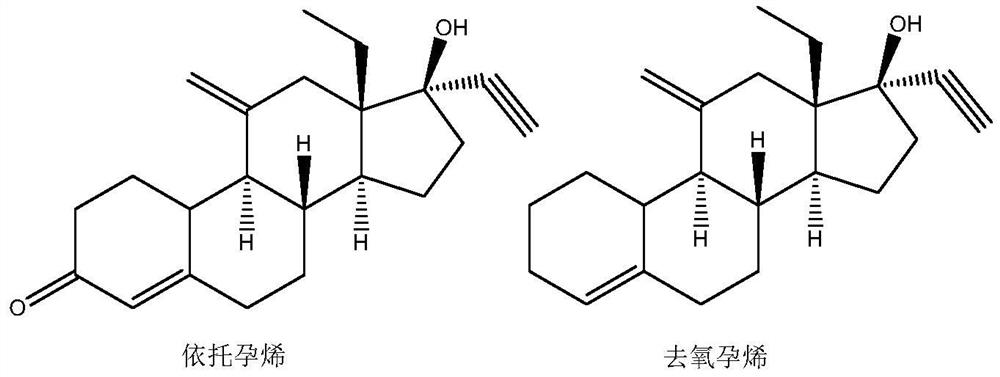
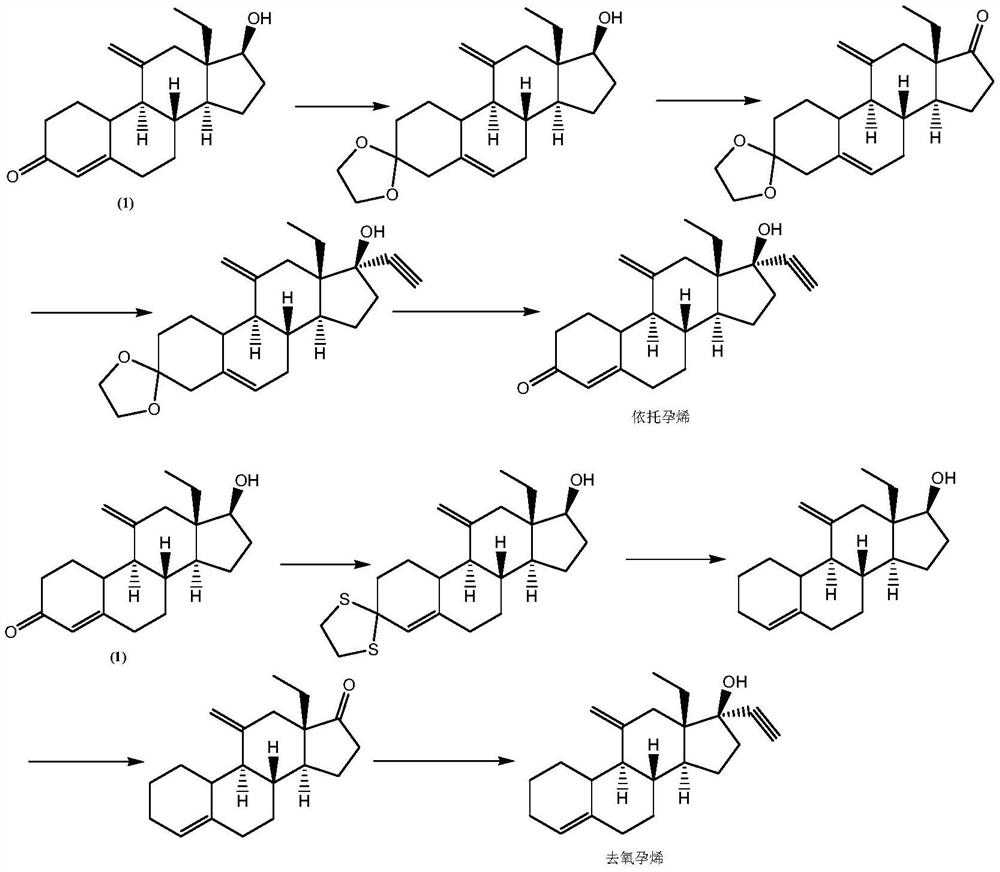
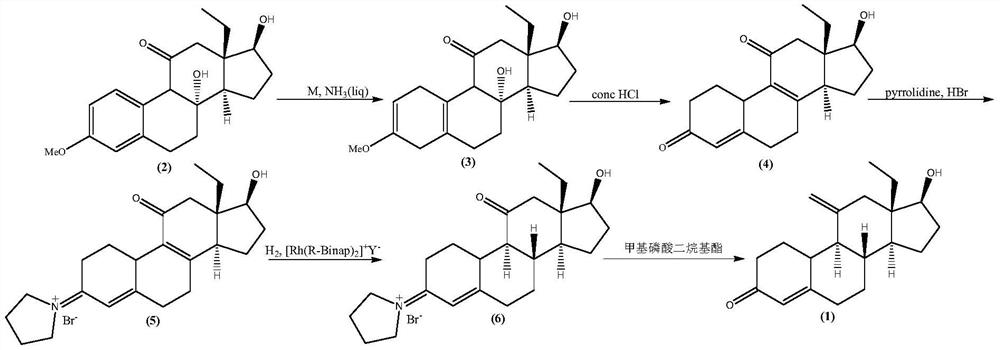
The preparation method of etonogestrel and desogestrel intermediate
A technology of etonogestrel and desogestrel, applied in the directions of steroids, organic chemistry, etc., can solve problems such as inability to obtain target products, and achieve the effects of easy industrialization, mild reaction conditions, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation of compound (3):
[0063] Pre-cool the system temperature to -50°C in a 500mL four-neck flask equipped with a thermometer, constant pressure dropping funnel, reflux condenser, and magnetic stirring in a dry nitrogen filled flask, then pass through barium oxide dry ammonia gas, and collect about 200mL After liquid ammonia, metal lithium particles (1.04 g; Fw: 6.94; 150.00 mmol) were gradually added in batches, and the system turned dark blue during the addition process. Then the compound of formula (2) (5.0g; Fw: 330.42; 15.13mmol) was dissolved in a mixed solvent of tetrahydrofuran and tert-butanol (50 ml of tetrahydrofuran, 10 ml of tert-butanol), and then the solution was slowly added dropwise to the system During the dropwise addition, the temperature of the system was maintained at -40°C. After the dropwise addition was completed, the system continued to stir for 0.5h.
[0064] After the reaction is complete, add solid ammonium chloride to the syste...
Embodiment 2
[0066] Preparation of compound (4):
[0067] Add 100 ml of methanol, 10.00 g of compound (3) and 5 ml of concentrated hydrochloric acid into a dry 250 mL three-neck flask equipped with a thermometer, a reflux condenser, and a magnetic stirrer filled with nitrogen, and then stir in the system at 20-25 °C for 1.5 h;
[0068] After the reaction is complete, add saturated sodium bicarbonate solution to the reaction system, adjust the pH to neutral, then add 100 milliliters of water, continue to stir until the crystals are completely precipitated, and then go through steps such as filtration, washing with water, and vacuum drying to obtain an off-white solid 9.04 grams, molar yield: 90%, HPLC content greater than 98.0%.
Embodiment 3
[0070] Preparation of compound (5):
[0071] Add 100 ml of methanol, compound (4) (10.00 g; Fw: 300.39; 33.30 mmol), tetrahydropyrrole (2.37 g; Fw: 71.12; 33.30mmol), then heated up to 50°C and stirred for 1.0h, a large amount of precipitation occurred;
[0072] Then the system was cooled to -20°C, and an aqueous solution of hydrobromic acid (5.61g, 48%, Fw: 80.91, 33.30mmol) was dissolved and slowly added dropwise to the reaction system;
[0073] After the dropwise addition, react at -20°C for 0.5h, then add 100ml of water, continue to stir until the crystals are completely precipitated, and then go through steps such as filtration, washing with water, and vacuum drying to obtain 13.67 grams of light yellow solid, molar yield: 94.5%, HPLC The content is greater than 97.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com