Preparation method of progesterone
A progesterone and reaction technology, applied in the directions of organic chemistry, steroids, organic chemistry, etc., can solve the problems of heavy metal ions, uneconomical atoms, cumbersome processes, etc., to improve the reaction yield, avoid high toxicity, and reduce the reaction high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
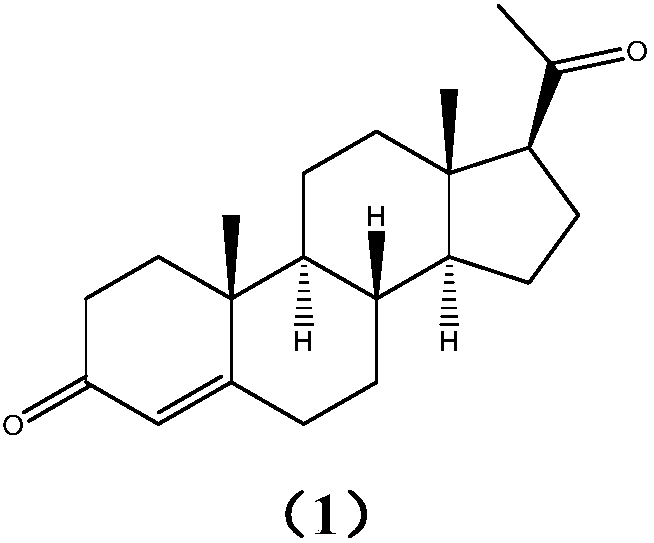
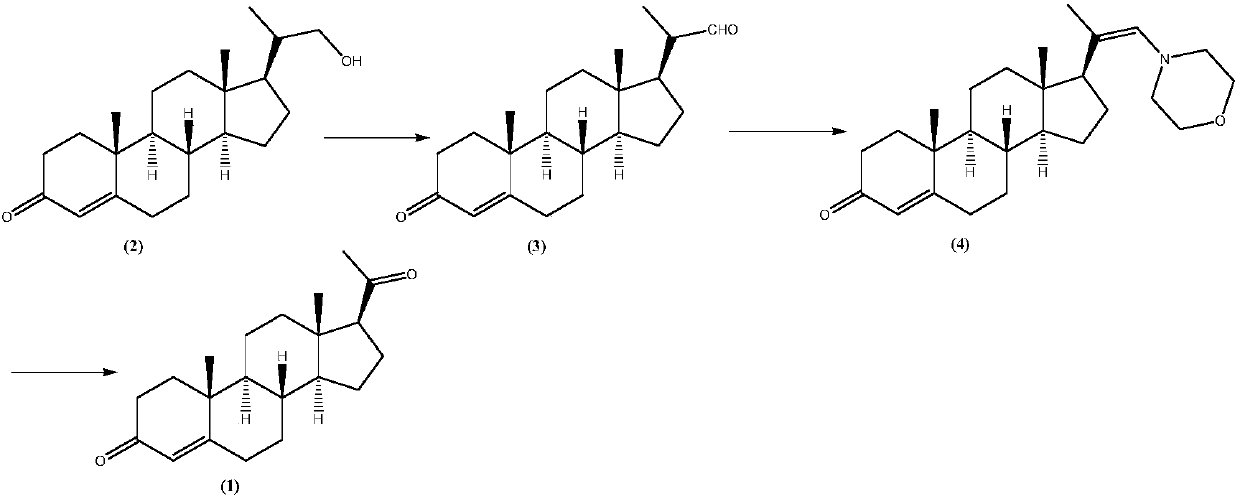
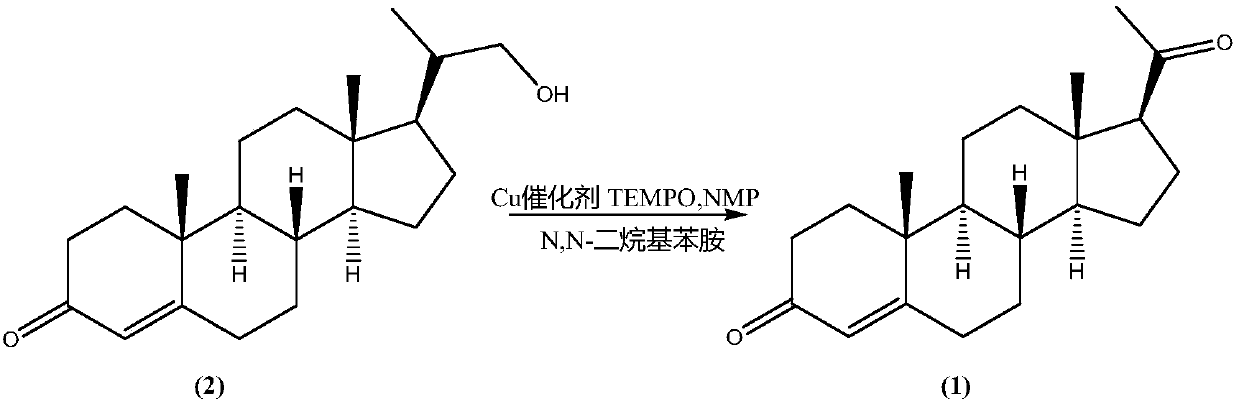
[0031] Preparation of Progesterone (1):
[0032] Compound (2) (16.53g; Fw: 330.50; 50mmol), methylene chloride 150mL, anhydrous copper acetate (0.091g) were added successively in a dry 250mL there-necked flask equipped with a thermometer, a reflux condenser and magnetic stirring and filled with nitrogen. ; Fw: 181.65; 0.5 mmol), NMP (0.050 g; Fw: 99.13; 0.5 mmol) TEMPO (0.078 g; Fw: 156.25; 0.5 mmol), N,N-dimethylaniline (0.061 g; Fw: 121.18; 0.5 mmol). The temperature of the system was then lowered to 0°C, and dry air was introduced. As the air was introduced into the system, the system gradually turned brown-red, and the temperature of the system was maintained at 0 °C during the air-introduction process. After feeding for about 12 hours, after the reaction was completed, 2M hydrochloric acid was slowly added dropwise to the reaction system, the pH was adjusted to neutrality, the organic phase was separated, the aqueous phase was extracted with dichloromethane, and then th...
Embodiment 2
[0034] Preparation of Progesterone (1):
[0035] Compound (2) (16.53g; Fw: 330.50; 50mmol), acetonitrile 150mL, anhydrous copper chloride (0.134g) were added successively in a dry nitrogen-filled 250mL there-necked flask equipped with a thermometer, reflux condenser, and magnetic stirring; Fw: 134.45; 1.0 mmol), NMP (0.10 g; Fw: 99.13; 1.0 mmol), TEMPO (0.16 g; Fw: 156.25; 1.0 mmol), N,N-dimethylaniline (0.12 g; Fw: 121.18; 1.0 mmol). The temperature of the system was then lowered to 0°C, and dry air was introduced. As the air was introduced into the system, the system gradually turned brown-red, and the temperature of the system was maintained at 0 °C during the air-introduction process. After feeding for about 12 hours, after the reaction was completed, 2M hydrochloric acid was slowly added dropwise to the reaction system, the pH was adjusted to neutrality, the organic phase was separated, the aqueous phase was extracted with dichloromethane, and then the organic phases we...
Embodiment 3
[0037] Preparation of Progesterone (1):
[0038] Compound (2) (16.53g; Fw: 330.50; 50mmol), acetonitrile 150mL, anhydrous copper fluoroborate (2.37g) were added successively in a dry nitrogen-filled 250mL there-necked flask equipped with a thermometer, reflux condenser, and magnetic stirring; Fw: 237.14; 10.0 mmol), NMP (1.00 g; Fw: 99.13; 10.0 mmol), TEMPO (1.56 g; Fw: 156.25; 10.0 mmol), N,N-dimethylaniline (1.21 g; Fw: 121.18; 10.0 mmol). The temperature of the system was then lowered to 0°C, and dry oxygen was introduced. With the introduction of oxygen into the system, the system gradually turned brown-red, and the system continued to exotherm during the introduction of oxygen, and the temperature of the system was maintained at 30 °C. After feeding for about 0.5h, after the reaction was completed, 2M hydrochloric acid was slowly added dropwise to the reaction system, the pH was adjusted to neutral, the organic phase was separated, the aqueous phase was extracted with d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com