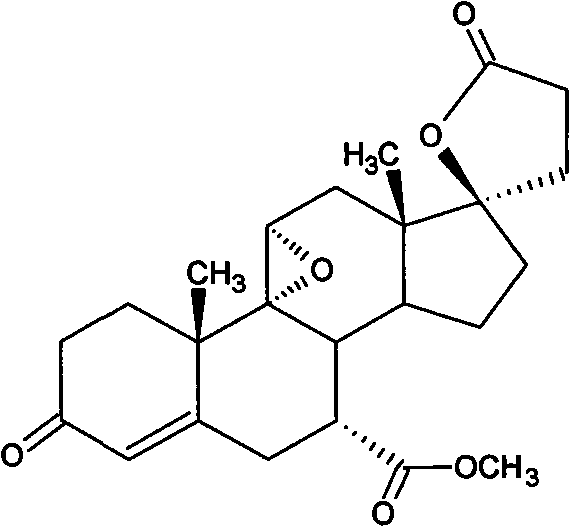
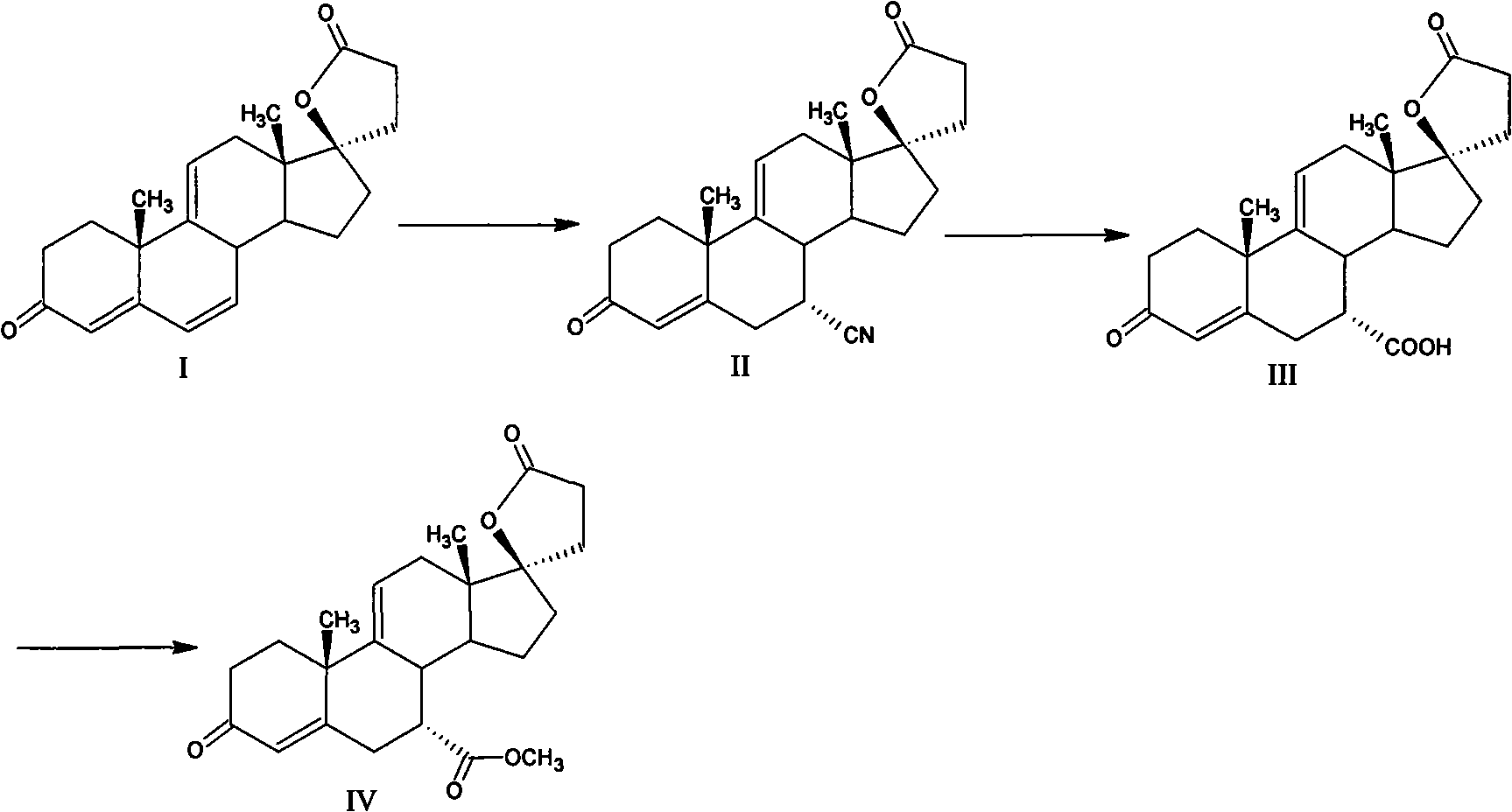
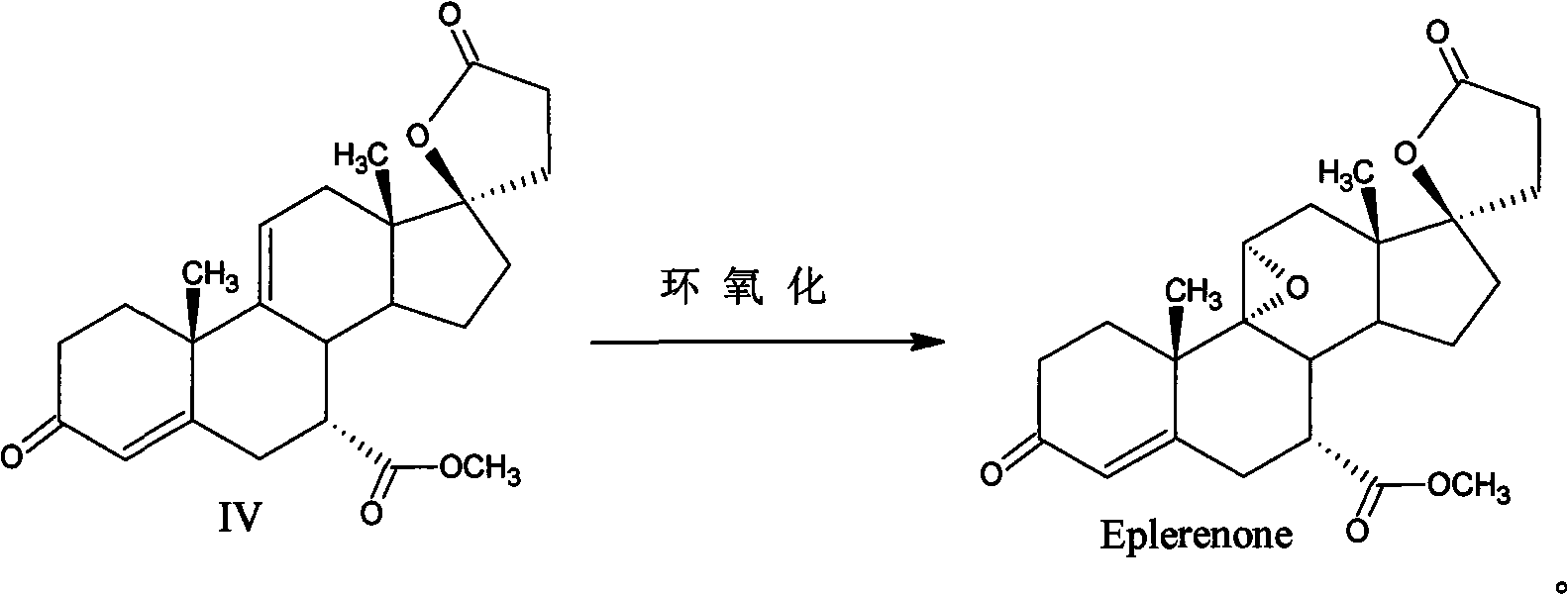
Method for preparing eplerenone
A technology of eplerenone and inhibitor is applied in the new preparation field of antihypertensive drug eplerenone, can solve the problems of controlling below 0.2%, unsuitable for large-scale production, not mentioned, etc., and the method is simple and feasible Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In a 250ml three-necked bottle with mechanical stirring and a thermometer, add 200ml of dichloromethane, 8.5g of dipotassium hydrogen phosphate, 15ml of trichloroacetonitrile, 1.5g of urea, dropwise add 75g of 30% hydrogen peroxide at room temperature, and heat the mixture under reflux for 4 hours. Cool to 30°C, then add 17α-hydroxyl-3-keto-γ-lactone-pregna-4,9(11)-diene-7α,21-dicarboxylate (formula IV) 10g, at 35 Heat the reaction at ~40°C, and then add 2ml of trichloroacetonitrile after 12 hours until the reaction is complete as detected by TLC. Separate the organic layer, back-extract the aqueous layer with 20ml of dichloromethane, combine the dichloromethane layers, wash with 3% sodium metabisulfite until non-oxidizing, and then wash with 100ml of water, dry the organic layer under reduced pressure, add 60ml of acetone to crystallize and filter, The crude product of eplerenone was obtained, and then recrystallized with methyl ethyl ketone to obtain 8.7 g of finished...
Embodiment 2
[0036] In a 250ml three-necked bottle with mechanical stirring and a thermometer, add 200ml of dichloromethane, 8.5g of dipotassium hydrogen phosphate, 15ml of trichloroacetonitrile, 0.75g of urea, dropwise add 75g of 30% hydrogen peroxide at room temperature, and heat the mixture under reflux for 4 hours. Cool to 30°C, then add 17α-hydroxyl-3-keto-γ-lactone-pregna-4,9(11)-diene-7α,21-dicarboxylate (formula IV) 10g, at 35 Heat the reaction at ~40°C, and add 2ml of trichloroacetonitrile after 12 hours until the reaction is complete as detected by TLC. Separate the organic layer, back-extract the aqueous layer with 20ml of dichloromethane, combine the dichloromethane layers, wash with 3% sodium metabisulfite until non-oxidizing, and then wash with 100ml of water, dry the organic layer under reduced pressure, add 60ml of acetone to crystallize and filter, The crude product of eplerenone was obtained, and then recrystallized with acetone to obtain 7.6 g of finished eplerenone with...
Embodiment 3
[0038] Add 200ml of dichloromethane, 8.5g of dipotassium hydrogen phosphate, 15ml of trichloroacetonitrile, 1.5g of thiourea into a 250ml three-neck flask with mechanical stirring and a thermometer, add 75g of 30% hydrogen peroxide dropwise at room temperature, and heat the mixture under reflux for 4 hours , cooled to 30°C, then added 17α-hydroxyl-3-keto-γ-lactone-pregna-4,9(11)-diene-7α,21-dicarboxylic acid methyl ester (formula IV) 10g, in Insulate the reaction at 35-40°C, and add 2ml of trichloroacetonitrile after 12 hours until the reaction is complete as detected by TLC. Separate the organic layer, back-extract the aqueous layer with 20ml of dichloromethane, combine the dichloromethane layers, wash with 3% sodium metabisulfite until non-oxidizing, and then wash with 100ml of water, dry the organic layer under reduced pressure, add 60ml of acetone to crystallize and filter, The crude product of eplerenone was obtained, and then recrystallized with butanone to obtain 8.2 g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com