A kind of canrenone derivative steroid compound, its preparation method and its application in the preparation of eplerenone
A technology of eplerenone and compounds, applied in the field of preparation of eplerenone, can solve the problems of high price, cumbersome operation steps, poor stereoselectivity, etc., and achieve easy scale-up production, high application value, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
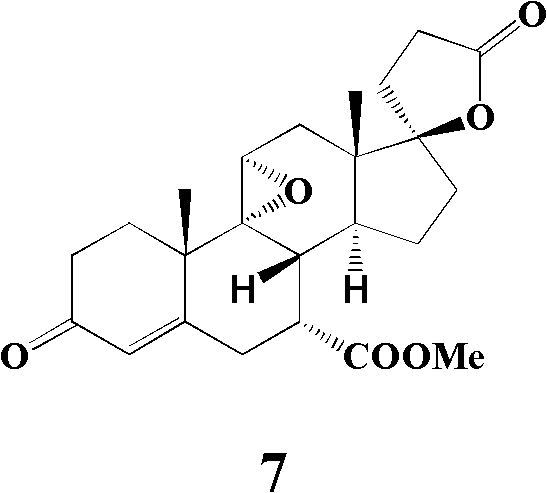
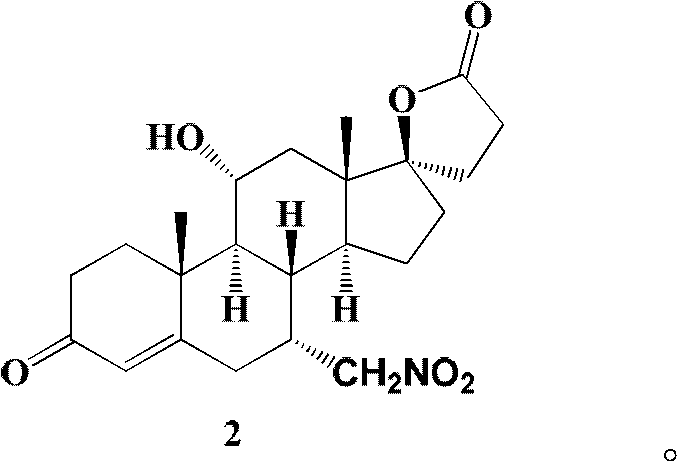
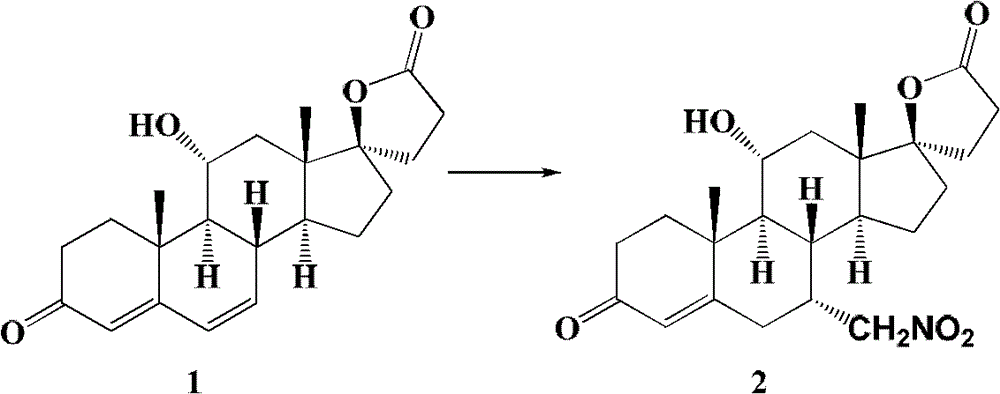
[0064] Embodiment 1: the preparation of the compound shown in formula 2
[0065]
[0066] Add compound (20g, 56mmol) shown in formula 1, quaternary ammonium salt (6mmol) (see Table 1), nitromethane (60ml, 112mmol) in the two-neck bottle that is connected with calcium chloride drying tube and air condenser and 190ml of N,N-dimethylformamide (DMF), after the dissolution is complete, the temperature is raised to 90-100°C, and K is added while stirring. 2 CO 3 (78g, 565mmol), kept fully stirred, and reacted at this temperature for 48 hours. When the reaction was terminated, the temperature was lowered first, and after the temperature was completely lowered to room temperature, it was filtered with diatomaceous earth, and the filter cake was washed with dichloromethane (100ml×3), and the dichloromethane layers were combined. A little 10% (w / w) dilute hydrochloric acid was added to adjust the pH value of the organic layer to 6. Spin all the solvents under reduced pressure firs...
Embodiment 2
[0078] Embodiment 2: the preparation of the compound shown in formula 4
[0079]
[0080] 1) The compound represented by Formula 2 (5 g, 0.012 mol) was dissolved in 40 ml of N,N-dimethylformamide (DMF), and then the temperature was raised to 45°C. Add acetic acid (2.9ml, 0.051mol) to this solution, after fully stirring, add NaNO in batches within 30min 2 (2.484g, 0.036mol), after adding NaNO 2Finally, let it react for one hour, after that, first mix acetic acid (2.9ml, 0.051mol) and 3mlDMF, and then add it dropwise to the above reaction solution. After the drop is completed, it takes about 4 hours. TLC analysis The raw material completely disappeared, and then the temperature was lowered to 0°C, and then saturated sodium sulfite solution (54ml, 0.089mol) was added to quench the reaction, and then the temperature was raised to room temperature, and the reaction was completely terminated overnight. After the reaction was completely terminated, all the solvents were spin-dri...
Embodiment 3
[0087] Embodiment 3: Preparation of the compound shown in formula 6 (R=methylsulfonyl)
[0088]
[0089] 1) At 0°C, the compound (4g, 9.6mmol) shown in formula 4 was dissolved in 54ml of dichloromethane solution, and then methanesulfonyl chloride (1.4ml, 17mmol) was added, followed by triethylamine ( 4.65ml, 31mmol) and 2ml of dichloromethane were mixed, and then added dropwise to the above reaction solution. After the dropwise addition was complete, the temperature was raised to room temperature and reacted for 3 hours. TLC detected that when the compound shown in formula 4 disappeared completely, the reaction was complete. completely. All the solvents were spin-dried to obtain the crude product of the compound represented by formula 5-1 as a white solid (reddish), which was directly used in the next step.
[0090] 2) Add acetic anhydride (1.8ml) dropwise to a solution of potassium formate (1.3g, 0.015mol) in formic acid (3.6ml) under nitrogen protection at room temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com