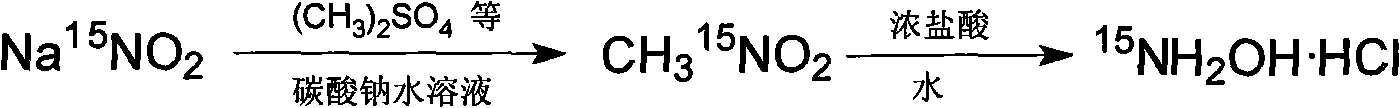Method for preparing N-hydroxylamine hydrochloride by hydrolyzing N-nitromethane
A technology for nitromethane and hydroxylamine hydrochloride, applied in the directions of hydroxylamine, nitrogen and non-metallic compounds, can solve the problems of complex synthesis steps, inability to large-scale production, high energy consumption, etc., and achieve easy availability of raw materials, product purity and abundance. And the effect of high yield and reasonable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A sort of 15 Preparation of N-nitromethane by hydrolysis 15 The method for N-hydroxylamine hydrochloride, the method may further comprise the steps:
[0027] (1) 15 Preparation of N-nitromethane
[0028] In a 500ml three-neck flask equipped with a magnetic stirrer, a thermometer and a dropping funnel, add an aqueous solution of anhydrous sodium carbonate (2g of anhydrous sodium carbonate dissolved in 20ml of water) and 8.8g (0.126mol) 15 N-sodium nitrite, the temperature of the mixture was lowered to 10°C, under vigorous stirring, 12ml (0.126mol) dimethyl sulfate was added dropwise within 30 minutes, after the addition was completed, the temperature was raised to 40°C, and reacted for 4h, Heating up and distilling under reduced pressure, the distilled liquid is separated by a water separator, and the lower layer is 15 N-nitromethane;
[0029] (2) 15 Preparation of N-hydroxylamine hydrochloride
[0030] In a 500ml three-neck flask equipped with magnetic stirring, ...
Embodiment 2
[0032] A sort of 15 Preparation of N-nitromethane by hydrolysis 15 The method for N-hydroxylamine hydrochloride, the method may further comprise the steps:
[0033] (1) 15 Preparation of N-nitromethane
[0034] In a 500ml three-neck flask equipped with magnetic stirring and a thermometer, add an aqueous solution of anhydrous sodium carbonate (2g of anhydrous sodium carbonate dissolved in 20ml of water) and 8.8g (0.126mol) 15 N-sodium nitrite, lower the temperature of the mixture to 5°C, under vigorous stirring, add 24g (0.252mol) chloroacetic acid within 30 minutes, after the addition, the temperature rises to 20°C, react for 6h, then heat up and distill under reduced pressure , the distilled liquid is separated by a water separator, and the lower layer is 15 N-nitromethane;
[0035] (2) 15 Preparation of N-hydroxylamine hydrochloride
[0036] In a 500ml three-neck flask equipped with magnetic stirring, thermometer and reflux condenser, add 6.83g (0.11mol) 15 N-nitrome...
Embodiment 3
[0038] A sort of 15 Preparation of N-nitromethane by hydrolysis 15 The method for N-hydroxylamine hydrochloride, the method may further comprise the steps:
[0039] (1) 15 Preparation of N-nitromethane
[0040] In a 500ml three-neck flask equipped with a magnetic stirrer, a thermometer and a dropping funnel, add an aqueous solution of anhydrous sodium carbonate (2g of anhydrous sodium carbonate dissolved in 20ml of water) and 8.8g (0.126mol) 15 N-sodium nitrite, the temperature of the mixture was lowered to 8°C, and under strong stirring, 32ml (0.252mol) methyl iodide was added dropwise within 30 minutes. Distillation under reduced pressure, the distilled liquid is separated by a water separator, and the lower layer is 15N-nitromethane;
[0041] (2) 15 Preparation of N-hydroxylamine hydrochloride
[0042] In a 500ml three-neck flask equipped with magnetic stirring, thermometer and reflux condenser, add 6.83g (0.11mol) 15 N-nitromethane, 5ml water and 24ml (0.279mol) co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com