Eplerenone oral solid preparation and preparation method therefor
A technology of eplerenone and solid preparations, applied in the field of medicine, can solve problems such as unfavorable water penetration and tablet disintegration, reduce liquid surface tension, increase insoluble drugs, etc., and achieve excellent preparation technical effects, solubility and The effect of dissolution enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
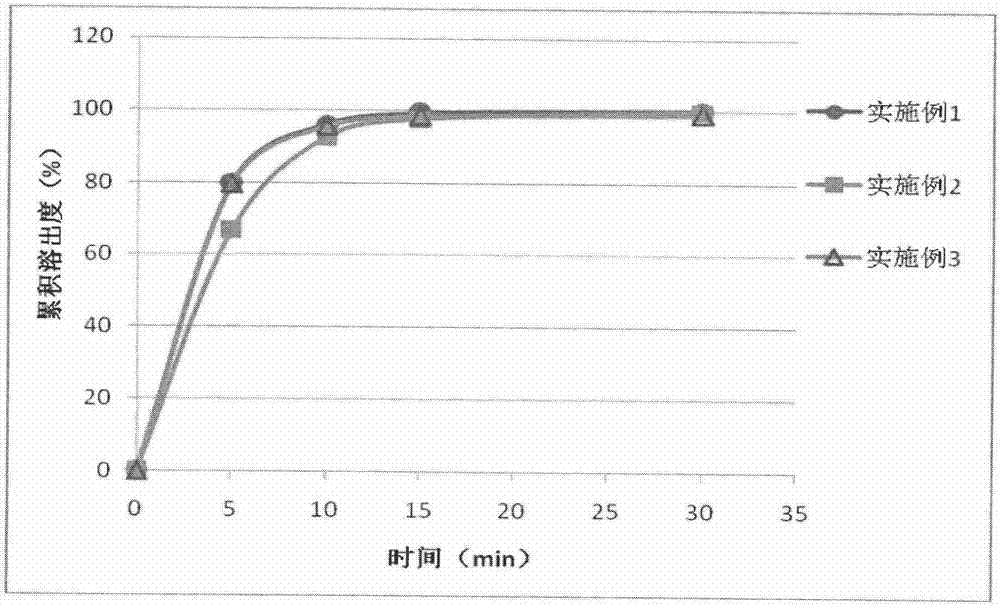
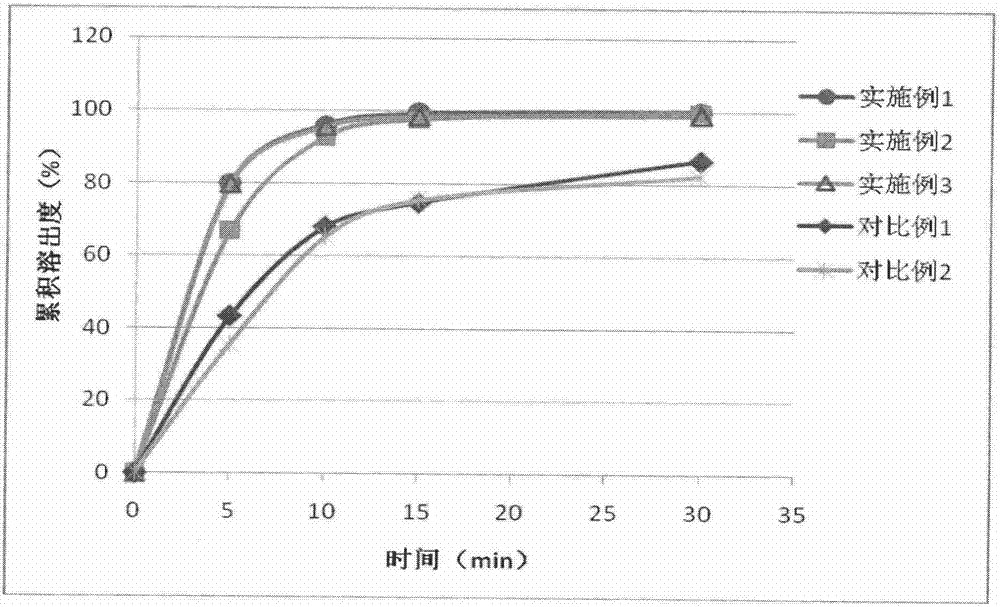
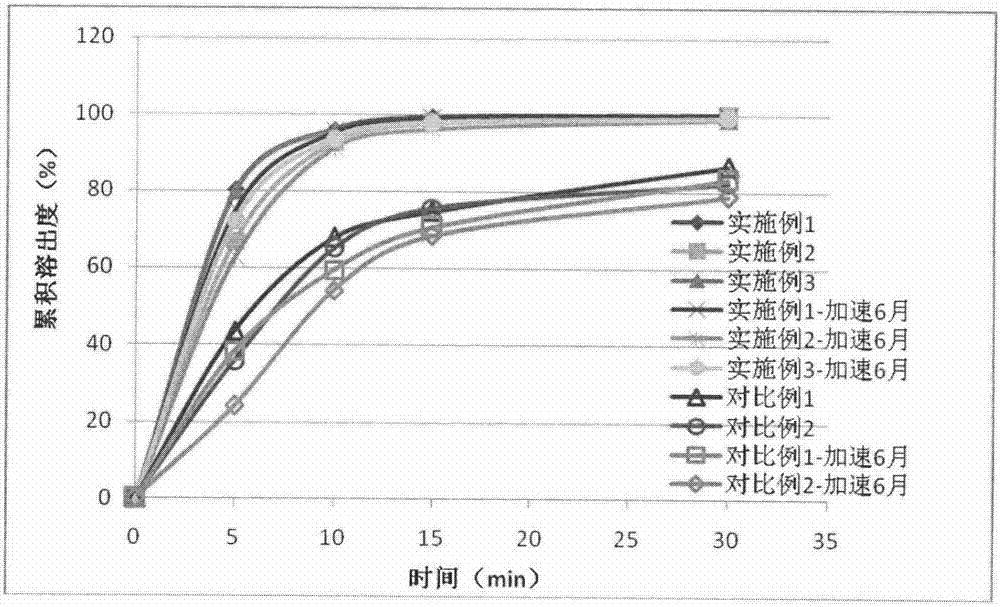
Embodiment 1
[0051] Eplerenone dispersible tablet or orally disintegrating tablet
[0052] prescription:
[0053]
[0054]
[0055] Preparation:
[0056] Wherein, the preparation method of eplerenone-polyvinylpyrrolidone compound:
[0057] a. Dissolve polyvinylpyrrolidone in ethanol, add eplerenone, and stir until dissolved under heating;
[0058] b. The solution of step a is granulated in one step by fluidized bed spray drying;
[0059] The preparation method of described dispersible tablet or capsule comprises:
[0060] (1) Grind the eplerenone-polyvinylpyrrolidone composite solid dispersion powder obtained by spray granulation with lactose, microcrystalline cellulose, and croscarmellose sodium, pass through a 40-mesh sieve and mix, add stearin Magnesium acid mixed evenly;
[0061] (2) Dry the mixed powder in an oven at 55°C until the drying loss is less than 2%, take it out and pass through a 40-mesh sieve, and press it into tablets. The tablet has a hardness of 5-10kg and a ...
Embodiment 2
[0063] Eplerenone Capsules
[0064] Components and dosage:
[0065]
[0066] Wherein, the preparation method of eplerenone-polyvinylpyrrolidone compound:
[0067] a. Dissolve polyvinylpyrrolidone in ethanol, add eplerenone, and stir until dissolved under heating;
[0068] b. The solution of step a is granulated in one step by fluidized bed spray drying;
[0069] The preparation method of described capsule comprises:
[0070] (1) The eplerenone-polyvinylpyrrolidone composite solid dispersion powder obtained by spray granulation is ground with lactose, microcrystalline cellulose, croscarmellose sodium and silicon dioxide, and mixed through a 40-mesh sieve ;
[0071] (2) Dry the mixed powder in an oven at 60°C until the drying loss is less than 2%, take it out and pass through a 40-mesh sieve, and fill it into capsules. The weight of the content of each capsule is about 170mg.
Embodiment 3
[0073] Eplerenone Tablets
[0074] prescription:
[0075]
[0076] Preparation:
[0077] Wherein, the preparation method of eplerenone-polyvinylpyrrolidone compound:
[0078] a. Dissolve polyvinylpyrrolidone in ethanol, add eplerenone, and stir until dissolved under heating;
[0079] b. The solution of step a is granulated in one step by fluidized bed spray drying;
[0080] The preparation method of described tablet comprises:
[0081] (1) Mix the eplerenone-polyvinylpyrrolidone composite solid dispersion powder obtained by spray granulation with lactose, microcrystalline cellulose, and hydroxypropyl cellulose evenly, add ethanol-made soft material, and pass the soft material through 40 mesh Sieve wet granules
[0082] (2) Place the wet granules in an oven at 55°C and dry until the loss on drying is less than 2%, take them out, pass through a 40-mesh sieve for granulation;
[0083] (3) Add croscarmellose sodium and magnesium stearate to the dry granules, mix well, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com