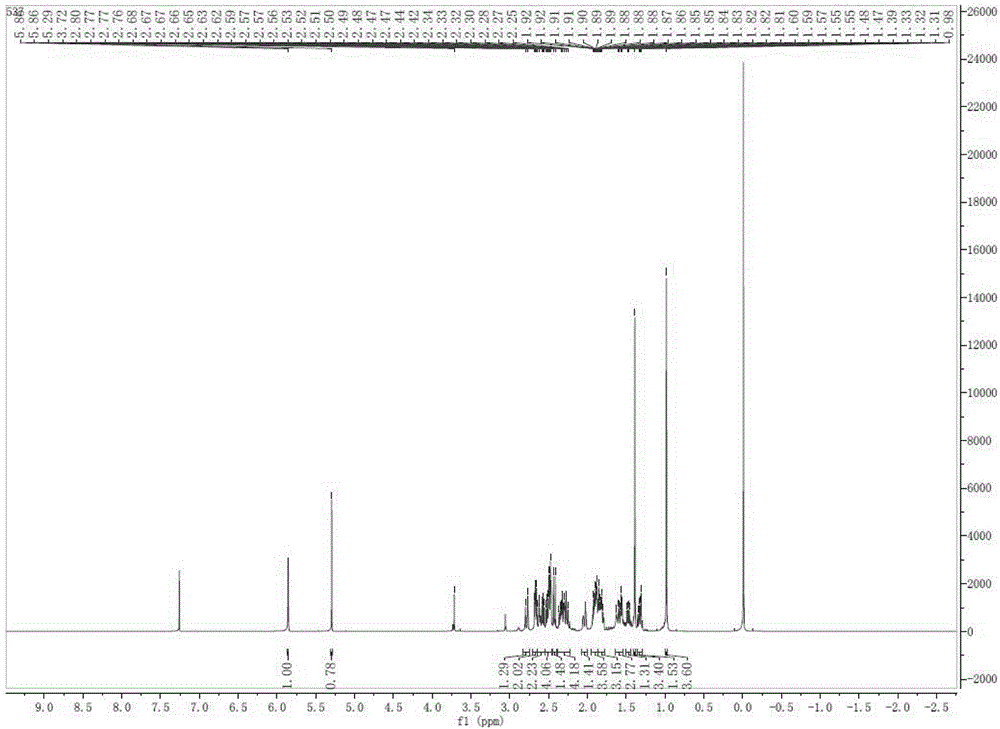
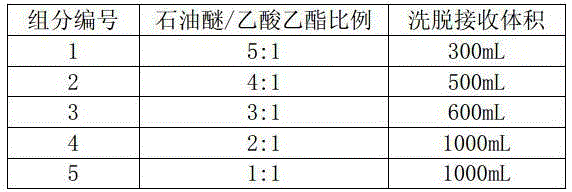
Method for preparing 17 alpha-hydroxyl-3-oxo-gamma-lactone-pregnene-4-alkene-(7 alpha, 9 alpha)-dicarboxylic acid lactone
A technology of dicarboxylic acid and lactone, applied in the direction of steroids, organic chemistry, etc., can solve the problems of complex process and low efficiency, and achieve the effect of simple process and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The first step, the synthesis of compound II;
[0042] Add compound I (10g, 0.024mol), triethylamine (6mL, 0.0432mol) and dichloromethane (60ml) into the three-necked flask, stir and dissolve, cool down to -10°C~-5°C, and maintain the temperature Slowly add methanesulfonyl chloride (3mL, 0.0388mol) dropwise. After the addition is complete, keep the reaction for 0.5~1h, and use thin-layer chromatography (TLC) to ensure that the raw materials are completely reacted, then concentrate under reduced pressure to dryness, and the oily yellow compound II is obtained. , the weight is about 11.6g, and the yield is 97.7%.
[0043] The second step, the synthesis of 17α-hydroxy-3-oxo-γ-lactone-pregn-4-ene-(7α, 9α)-dicarboxylic acid lactone;
[0044] Add 50mL of acetic acid / water (1:4) and sodium acetate (5g, 0.0609mol) into a three-neck flask, raise the temperature to 40°C-50°C, stir for 30min, then add compound II (10g, 0.0202mol) into it, and raise the temperature to 110°C, keep...
Embodiment 2
[0056] The first step, the synthesis of compound II:
[0057] Add compound I (10g, 0.024mol), triethylamine (6mL, 0.0432mol) and chloroform (60ml) into the three-necked flask, stir and dissolve, cool down to -10°C~-5°C, and slowly drop Add methanesulfonyl chloride (3mL, 0.0388mol). After the addition, keep it warm for 0.5~1h, and use TLC method to ensure that the raw materials are completely reacted, then concentrate under reduced pressure and close to dryness to obtain 10.64g of oily yellow compound II, with a yield of 93.8 %.
[0058] The second step, the synthesis of compound A:
[0059] Add 40mL of acetic acid / water (1:3) and sodium hydroxide (4g, 0.099mol) into a three-necked flask, raise the temperature to 40~50°C, stir for 30min, then add compound II (10g, 0.0202mol), and raise the temperature to 110 ~120°C, keep warm for 6 hours, and use TLC method to ensure the complete reaction of the raw materials. After cooling to 25°C, add 30mL of dichloromethane and 30mL of wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com