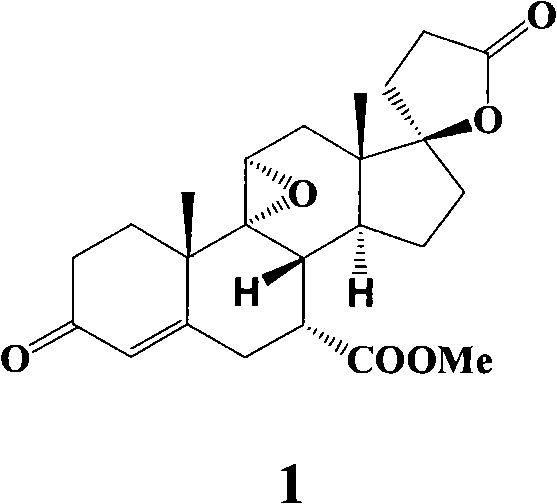
Preparation method of eplerenone and intermediate thereof
A technology of acetone and compounds, which is applied in the field of medicine and can solve the problems of high price and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
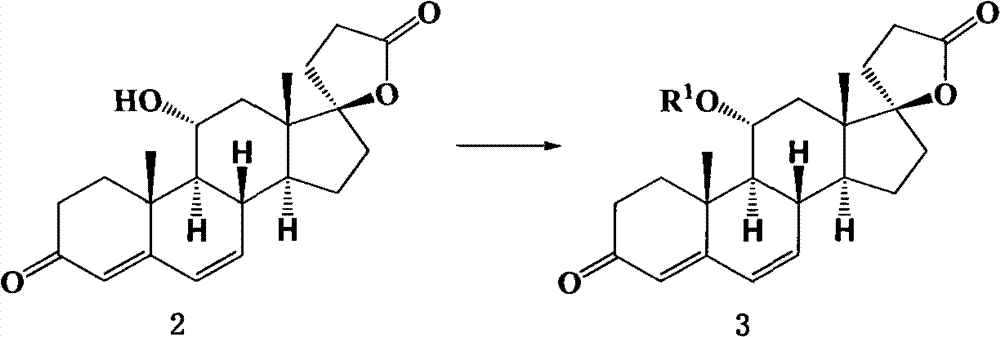
[0110] Embodiment 1: the preparation of compound shown in formula 3 (R 1 = methylsulfonyl)
[0111]
[0112] The compound shown in Formula 2 (5.800g, 0.0163mol) and triethylamine (2.29ml, 0.0163mol) were dissolved in 55ml of dichloromethane, cooled to 0°C in an ice-water bath, and methylsulfonate was added dropwise at this temperature Acyl chloride (1.26ml, 0.0180mol) was recrystallized from acetone / dichloromethane to obtain the compound represented by formula 3 (colorless transparent crystals), with a yield of 99%.
[0113] mp: 188~190℃;
[0114] 1 H NMR (300MHz, CDCl 3 )δ6.16(dd, J=9.7, 2.2Hz, 1H), 6.00(d, J=9.7Hz, 1H), 5.71(s, 1H), 5.15(td, J=10.4, 4.9Hz, 1H), 3.02(s, 3H), 1.28(s, 3H), 1.09(s, 3H)
[0115] 13 C NMR (300MHz, CDCl 3 )δ 199.05, 176.00, 161.20, 137.06, 129.09, 125.53, 94.30, 77.80, 53.40, 46.43, 46.41, 40.84, 40.20, 37.60, 36.67, 35.40, 34.78, 34.23, 17, 30.957, 5.3, 22.5
Embodiment 2
[0116] Embodiment 2: the preparation of compound shown in formula 4
[0117]
[0118] Potassium formate (6.655g, 0.079mol), formic acid (150ml), and acetic anhydride (7.48ml, 0.079mol) were mixed at room temperature and heated to 80°C for 12 hours. After that, the compound represented by formula 3 (34.329g, 0.079mol) was added, and the temperature was raised to 100°C to react for 3 hours. After most of the solvent was spin-dried under reduced pressure, dichloromethane and water were added for extraction, and the organic layers were combined, and the organic layers were successively washed with saturated NaHCO 3 Washed three times with aqueous solution, water, and saturated brine, and separated by column chromatography to obtain the compound described in Formula 4 (white yellowish solid powder), with a yield of 90%.
[0119] 1 H NMR (300MHz, CDCl 3 )δ6.16(dd, J=9.86, 2.19Hz, 1H), 6.09(dd, J=9.65, 1.31Hz, 1H), 5.69(s, 1H), 5.55-5.48(m, 1H), 1.30(s , 3H), 0.99 (s, 3H).
[...
Embodiment 3
[0121] Embodiment 3: directly prepare the compound shown in formula 4 by the compound shown in formula 2
[0122]
[0123] At -50°C, compound 2 (116mg, 0.33mmol), tetrahydrofuran (20ml), dimethyl sulfoxide (39mg, 0.33mmol) and NaCl (20mg, 0.33mmol) were sequentially added, stirred thoroughly and gradually warmed to room temperature, Water (20 ml) was added to terminate the reaction, and extracted with ethyl acetate to obtain 89 mg of the compound represented by formula 4, with a yield of 80%.
[0124] 1 H NMR (300MHz, CDCl 3 )δ6.16(dd, J=9.86, 2.19Hz, 1H), 6.09(dd, J=9.65, 1.31Hz, 1H), 5.69(s, 1H), 5.55-5.48(m, 1H), 1.30(s , 3H), 0.99 (s, 3H).
[0125] 13 C NMR (300MHz, CDCl 3 )δ199.065,176.36,161.827,140.325,137.854,127.257,123.895,118.501,94.794,44.276,43.962,38.835,38.538,35.212,34.024,32.878,32.023,31.174,29.075,24.354,22.877,14.318。
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com