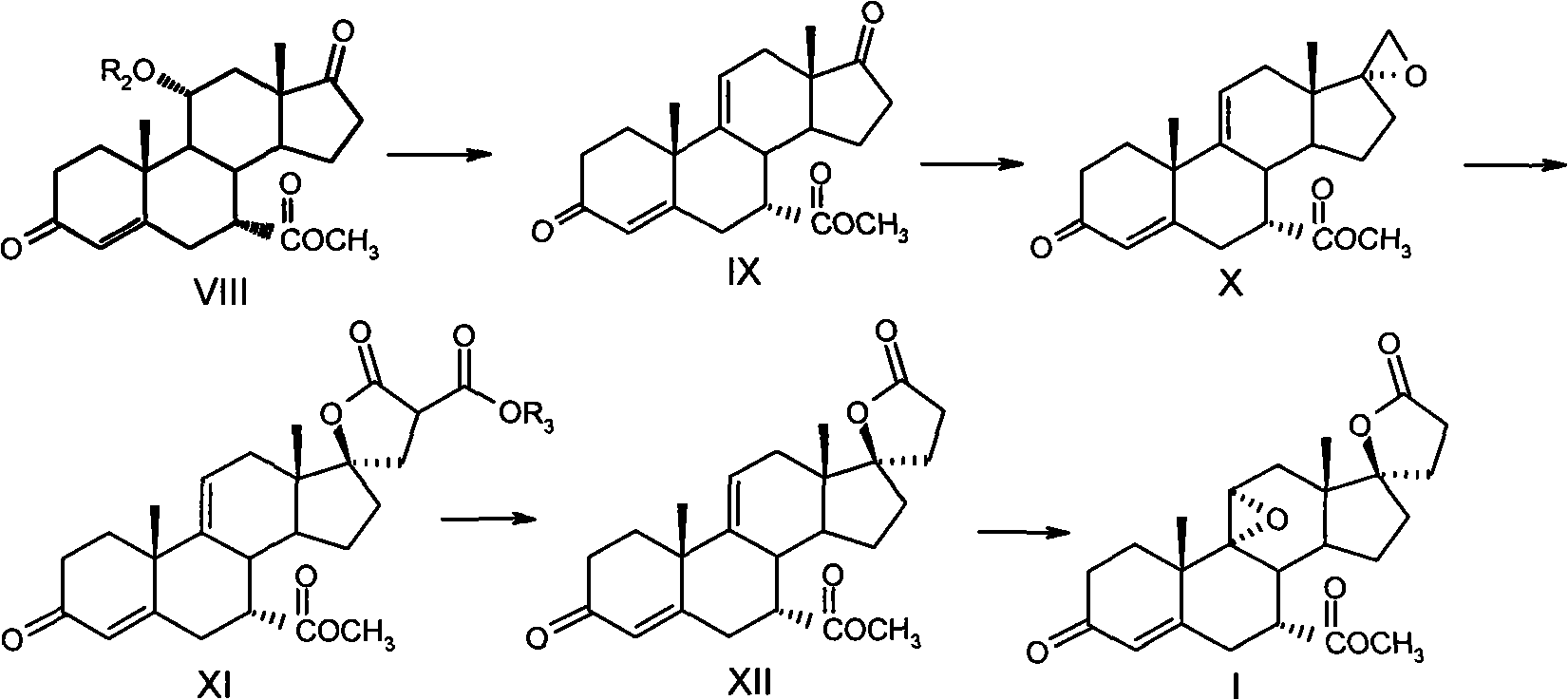
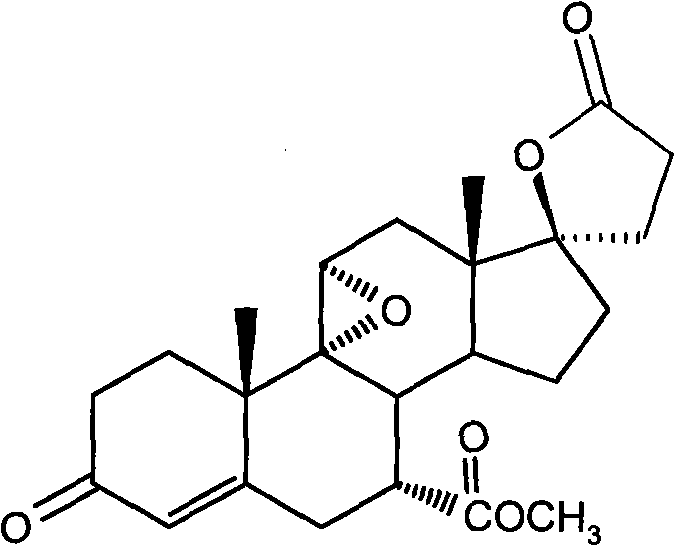
Method for synthesizing eplerenone
A technology of eplerenone and ketone group is applied in the field of synthesizing steroidal antihypertensive drug eplerenone, and can solve the problems of high market price of canrenone, difficult process control, low reaction yield and the like, Achieve the effect of large industrial production value, easy control of process operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
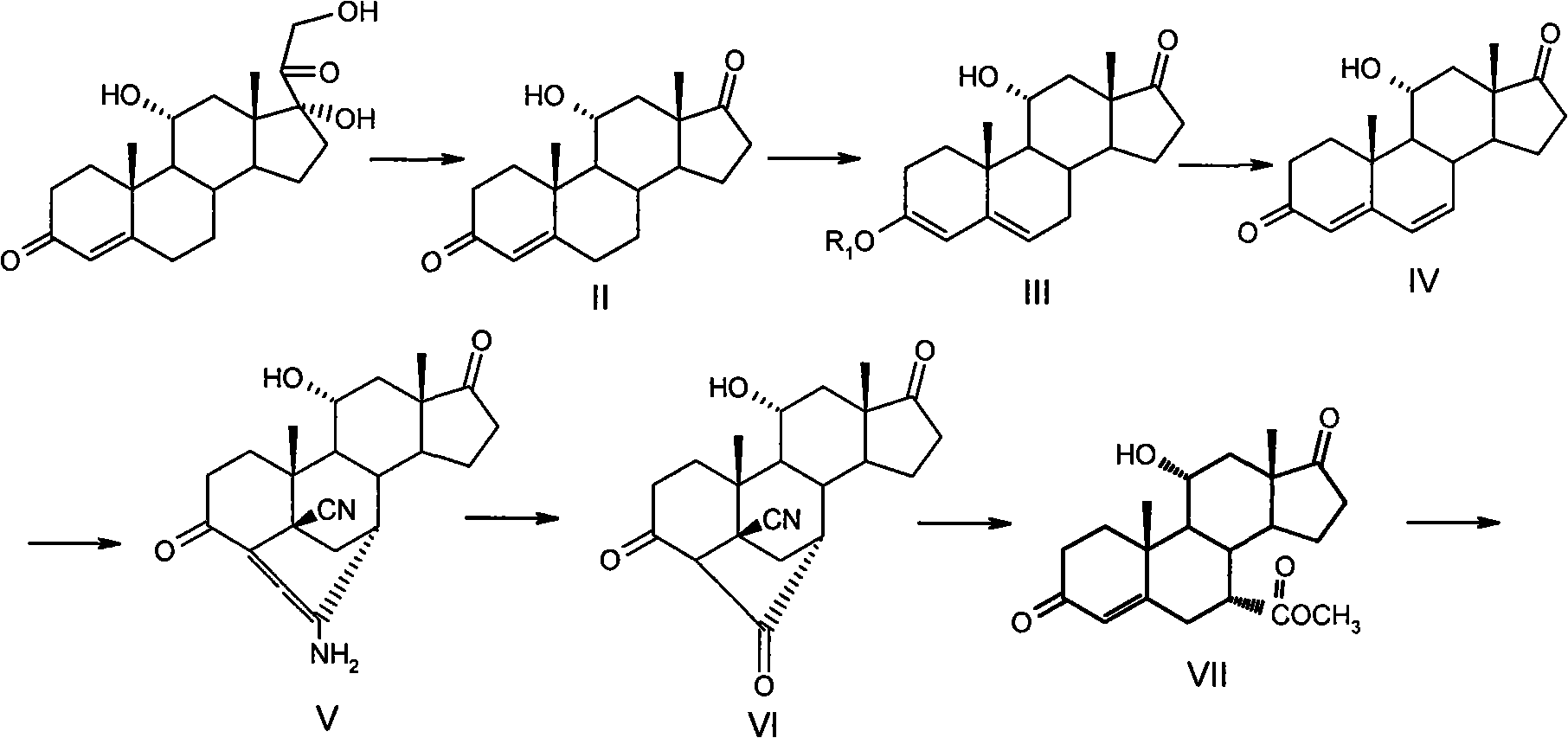
[0045] 4-ene-11α-hydroxy-3,17-dione-androstane (II)
[0046] Under argon protection, 10.0 g of epicortisol was added to 400 ml of ethyl acetate, 40.0 g of manganese dioxide was added, and the mixture was heated to reflux for 3 hours. Filter while hot. After the filter cake was washed with 30 ml of ethyl acetate, 60 ml of ethyl acetate was added and the mixture was stirred and refluxed for 30 minutes. repeat. The obtained filtrates were combined and concentrated to dryness under reduced pressure to obtain 6.4 g of pale yellow crude product. The crude product was refluxed for half an hour by adding 10 ml of methanol, and crystallized by freezing. Filter and dry to obtain 5.0 g of product (II).
Embodiment 2
[0048] 3-Ethoxy-3,5(6)-diene-11α-hydroxy-17-one-androstane(III)
[0049] Under argon protection, 10.0 g of the compound of formula II was dissolved in 350 ml of dioxane, and then 34 ml of triethyl orthoformate, 0.6 g of p-toluenesulfonic acid and 10 ml of absolute ethanol were sequentially added. The reaction temperature was raised to 40°C, and the reaction was incubated for 1.5 hours. Followed by TLC (cyclohexane:ethyl acetate=3:1) until the reaction was complete, 140ml of saturated NaHCO was added rapidly 3 The solution terminates the reaction, the reaction solution is poured into 1 L of water, 300 ml of dichloromethane is added, stirred and left to separate layers. The separated aqueous phase was extracted with dichloromethane (50 x 3) to no product. The organic layers were combined, washed with saturated NaCl until neutral, anhydrous Na 2 SO 4 dry. After filtration, the filtrate was concentrated to dryness to give a yellow solid. The crude product was purified by rec...
Embodiment 3
[0051] 4,6-Diene-11α-hydroxy-3,17-dione-androsta(IV)
[0052] 10.0 g of the compound of formula III was dissolved in 70 ml of acetone, stirred, and heated to 30-35°C. After all the solids were dissolved, 25 ml of water was added to separate out the solids, the temperature was lowered to 25-30° C., the reaction system was protected by argon gas, 8.0 g of tetrachlorobenzoquinone was added in the dark, and the reaction was maintained for about 1.5 hours. After the reaction was completed, it was poured into 210 ml of 10% NaOH solution and stirred for 0.5 hour. filter. The filter cake was washed with 50 ml of dichloromethane. The filtrates were combined and extracted with dichloromethane (50 ml x 4). The dichloromethane layer was successively washed with saturated Na 2 8 2 O 3 Aqueous solution and water wash. Anhydrous Na 2 SO 4 dry. filter. The filtrate was concentrated to dryness to obtain a yellow crude product. The crude product was purified with isopropyl ether to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com