Oral liquid-crystal sustained-release composition and preparation
A slow-release composition and liquid crystal technology, applied in the direction of heterocyclic compound active ingredients, drug delivery, medical preparations of non-active ingredients, etc., can solve problems affecting drug release, blood drug concentration fluctuations, preparation toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
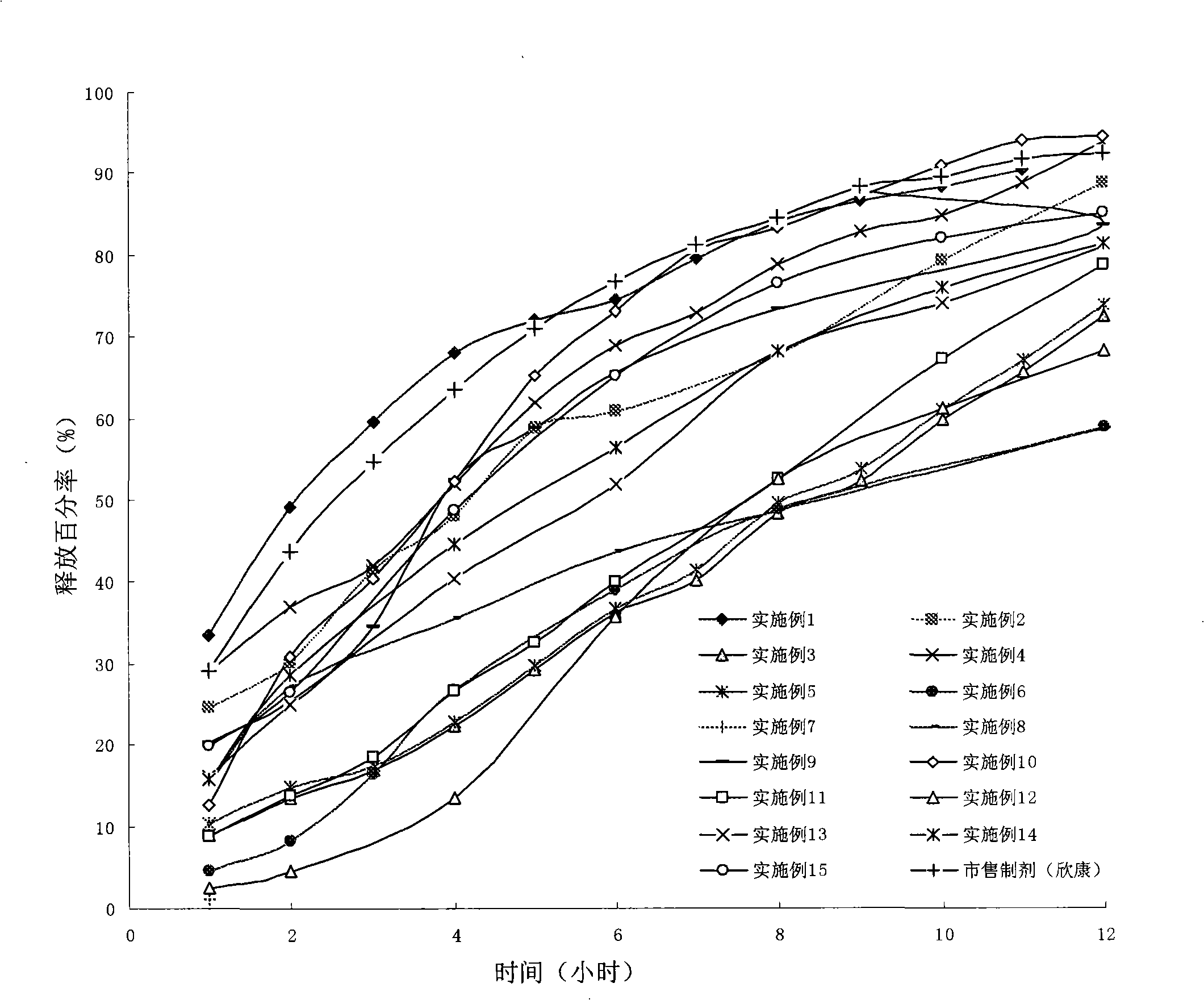
Image
Examples
Embodiment 1
[0122] The formula of liquid crystal sustained-release composition (sustained-release capsule) 1
[0123] Preparation Content of ingredients in each tablet (mg)
[0124] Isosorbide Mononitrate 40mg
[0125] Glyceryl Monooleate 420mg
[0126] Compritol ATO 888 25mg
[0127] Gelucire 43 / 01 25mg
[0128] Preparation:
[0129] Heat glyceryl monooleate to 45°C to melt, add isosorbide mononitrate and stir to dissolve under stirring, then add Compritol ATO888 and Gelucire 43 / 01, stir well, cool to about 40°C, fill in hard capsule shell In the process, each capsule is made into sustained-release capsules containing 40 mg of isosorbide mononitrate.
Embodiment 2
[0131] The formula of liquid crystal sustained-release composition (sustained-release capsule) 2
[0132] Preparation Content of ingredients in each tablet (mg)
[0133] Acemetacin 90mg
[0134] Glyceryl Monooleate 400mg
[0135] Compritol ATO 888 40mg
[0136] Gelucire 40 / 13 18mg
[0137] Preparation:
[0138] Heat glyceryl monooleate to 50°C to melt, add acemetacin under stirring, then add Compritol ATO 888 and Gelucire40 / 13, stir evenly, cool to about 40°C, fill in soft capsules, and make Each capsule contains acemetacin 90mg sustained-release capsules.
Embodiment 3
[0140] The formula of liquid crystal sustained-release composition (sustained-release capsule) 3
[0141] Preparation Content of ingredients in each tablet (mg)
[0142] Gliclazide 30mg
[0143] Glyceryl Monooleate 250mg
[0144] Compritol ATO 888 20mg
[0145] Polysorbate 80 15mg
[0146] Preparation:
[0147] Heat glyceryl monooleate to 60°C to melt, add gliclazide under stirring, then add Compritol ATO 888 and polysorbate 80, stir evenly, cool to about 40°C, fill in the capsule shell, and make Formed into sustained-release capsules containing 30 mg of gliclazide in each capsule.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com