Levocetirizine dihydrochloride granule and preparation and detection methods thereof
A technology of levocetirizine hydrochloride and detection method, which is applied in the direction of pharmaceutical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as poor adhesion, incomplete absorption, and inconvenience for children to take. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
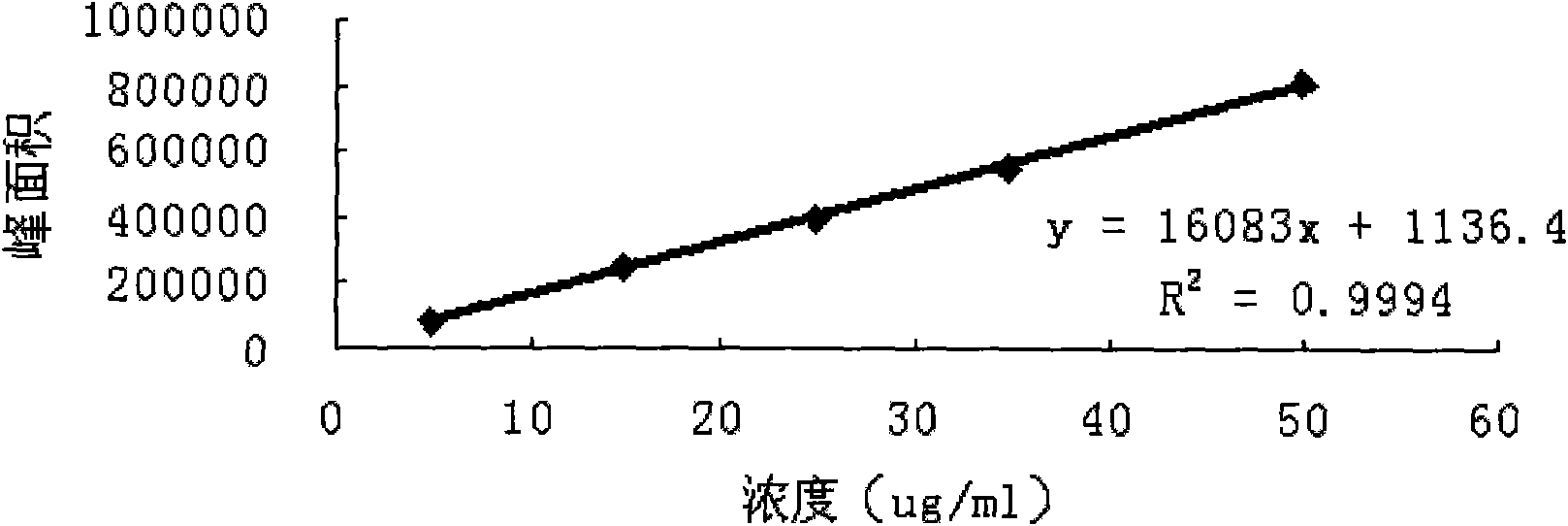
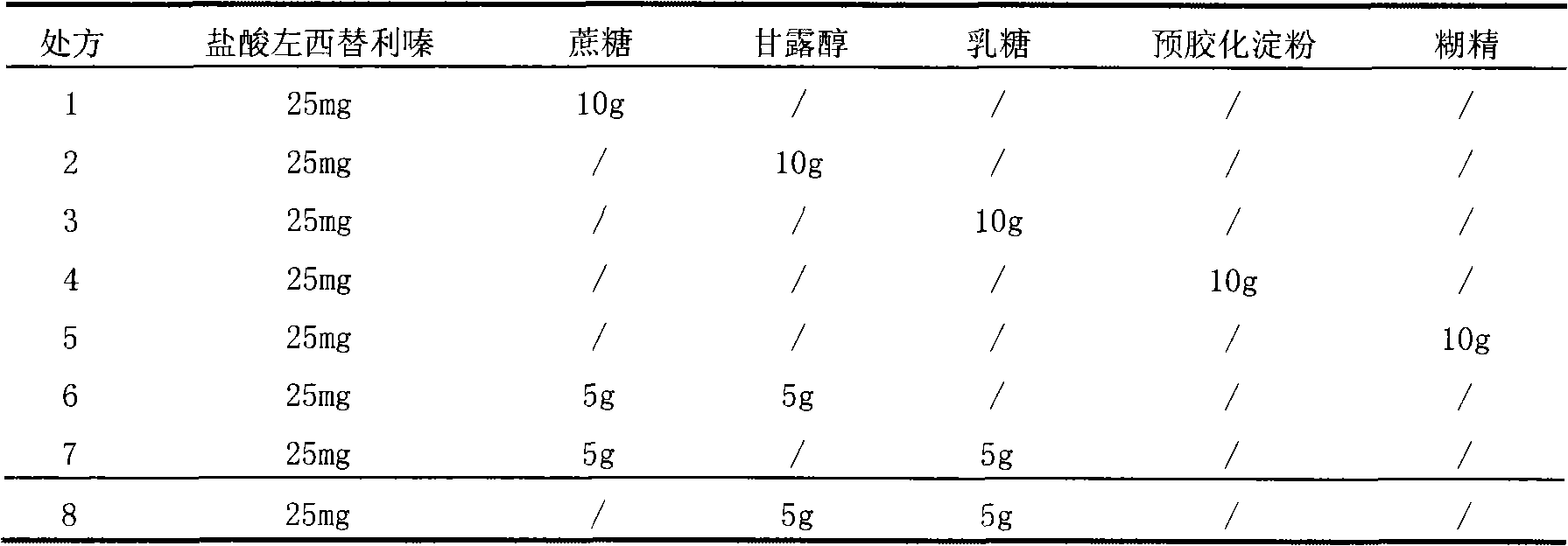
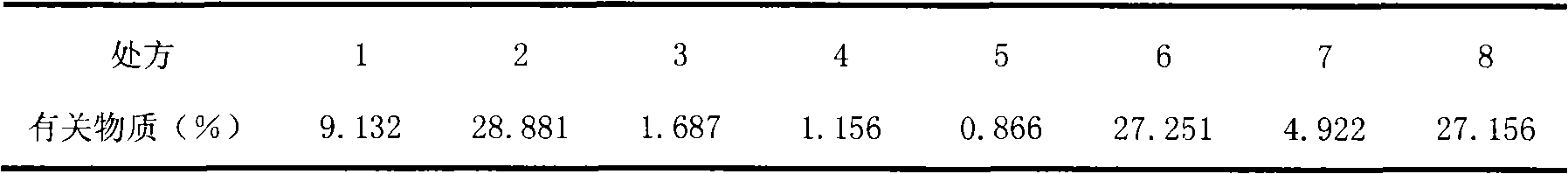
Image
Examples
Embodiment 1
[0170] Embodiment 1: Levocetirizine hydrochloride granules
[0171] Each bag contains 2.5 mg of levocetirizine hydrochloride, and the raw material composition of 1000 bags of levocetirizine hydrochloride granules is:
[0172] Levocetirizine Hydrochloride 2.5g
[0173] Lactose 1000g
[0174] Aspartame 2.0g
[0175] Povidone K30 20g
[0176] a, pulverize levocetirizine hydrochloride through an 80-mesh sieve for subsequent use; lactose and aspartame are respectively passed through an 80-mesh sieve for subsequent use; povidone K30 is made into a 10% aqueous solution for subsequent use;
[0177] b. Weigh the lactose, aspartame, levocetirizine hydrochloride of prescription amount, after aspartame, levocetirizine hydrochloride mix uniformly, then mix homogeneously with lactose according to the principle of increasing in equal quantity to obtain the mixture ;
[0178] c, transfer the mixture to the blender, use 10% povidone K30 aqueous solution as a binder to make a soft material...
Embodiment 2
[0182] Embodiment 2: Levocetirizine hydrochloride granule
[0183] Each bag contains 2.5 mg of levocetirizine hydrochloride, and the raw material composition of 1000 bags of levocetirizine hydrochloride granules is:
[0184] Levocetirizine Hydrochloride 2.5g
[0185] Lactose 600g
[0186] Aspartame 1.0g
[0187] Povidone K30 12g
[0188] a, pulverize levocetirizine hydrochloride through a 100-mesh sieve for subsequent use; lactose and aspartame are respectively passed through a 100-mesh sieve for subsequent use; povidone K30 is made into a 15% aqueous solution for subsequent use;
[0189] b. Weigh the lactose, aspartame, levocetirizine hydrochloride of prescription amount, after aspartame, levocetirizine hydrochloride mix uniformly, then mix homogeneously with lactose according to the principle of increasing in equal quantity to obtain the mixture ;
[0190] c, transfer the mixture to the blender, use 15% povidone K30 aqueous solution as a binder to make a soft material, ...
Embodiment 3
[0194] Embodiment 3: Levocetirizine hydrochloride granule
[0195] Each bag contains 2.5 mg of levocetirizine hydrochloride, and the raw material composition of 1000 bags of levocetirizine hydrochloride granules is:
[0196] Levocetirizine Hydrochloride 2.5g
[0197] Lactose 1400g
[0198] Aspartame 2.8g
[0199] Povidone K30 28g
[0200] a. Grinding levocetirizine hydrochloride through a 60-mesh sieve for subsequent use; lactose and aspartame were respectively passed through a 60-mesh sieve for subsequent use; Povidone K30 was made into a 7% aqueous solution for subsequent use;
[0201] b. Weigh the lactose, aspartame, levocetirizine hydrochloride of prescription amount, after aspartame, levocetirizine hydrochloride mix uniformly, then mix homogeneously with lactose according to the principle of increasing in equal quantity to obtain the mixture ;
[0202] c, transfer the mixture to the blender, use 7% povidone K30 aqueous solution as a binder to make a soft material, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com