Porous paclitaxel matrices and methods of manufacture thereof
a technology of paclitaxel and matrices, which is applied in the field of paclitaxel formulations, can solve the problems of difficult dosage forms and difficulty in providing, and achieve the effects of reducing volume, prolonging half-life, and fast dissolution ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of a Porous Paclitaxel Matrix Using Ammonium Bicarbonate as a Pore Forming Agent
[0064]A paclitaxel-loaded organic solution was prepared by dissolving 1.0 g of paclitaxel, 0.10 g of TWEEN™ 80, and 0.10 g of polyvinylpyrrolidone K-15 in 160 ml of ethanol. An aqueous solution composed of 0.42 g of ammonium bicarbonate and 1.0 g of mannitol in 40 ml of DI water was added to the ethanol solution and mixed. The resulting 80% ethanol solution was spray dried on a benchtop spray dryer using an air-atomizing nozzle and nitrogen as the drying gas. Spray drying conditions were as follows: 20 ml / min solution flow rate, 60 L / min atomization gas rate, 100 kg / hr drying gas rate, and 55° C. outlet.
example 2
Production of a Porous Paclitaxel Matrix Using Ammonium Bicarbonate as a Pore Forming Agent
[0065]A paclitaxel-loaded organic solution was prepared by dissolving 0.4 g of paclitaxel, 0.10 g of TWEEN™ 80, and 0.04 g of polyvinylpyrrolidone K-15 in 160 ml of ethanol. An aqueous solution composed of 0.30 g of ammonium bicarbonate and 1.0 g of mannitol in 40 ml of DI water was added to the ethanol solution and mixed. The resulting 80% ethanol solution was spray dried on a benchtop spray dryer using an air-atomizing nozzle and nitrogen as the drying gas. Spray drying conditions were as follows: 20 ml / min solution flow rate, 60 L / min atomization gas rate, 100 kg / hr drying gas rate, and 55° C. outlet temperature.
example 3
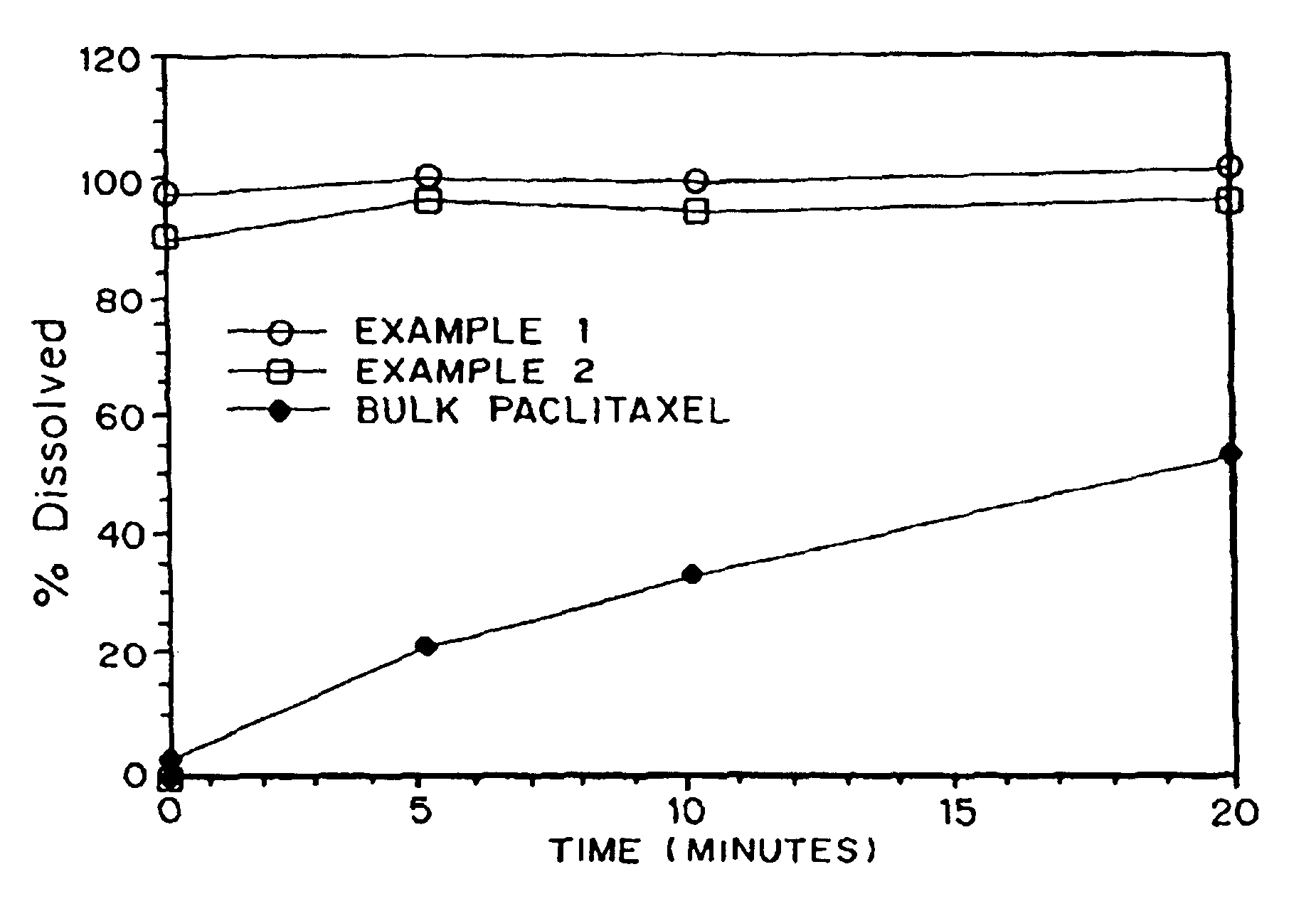
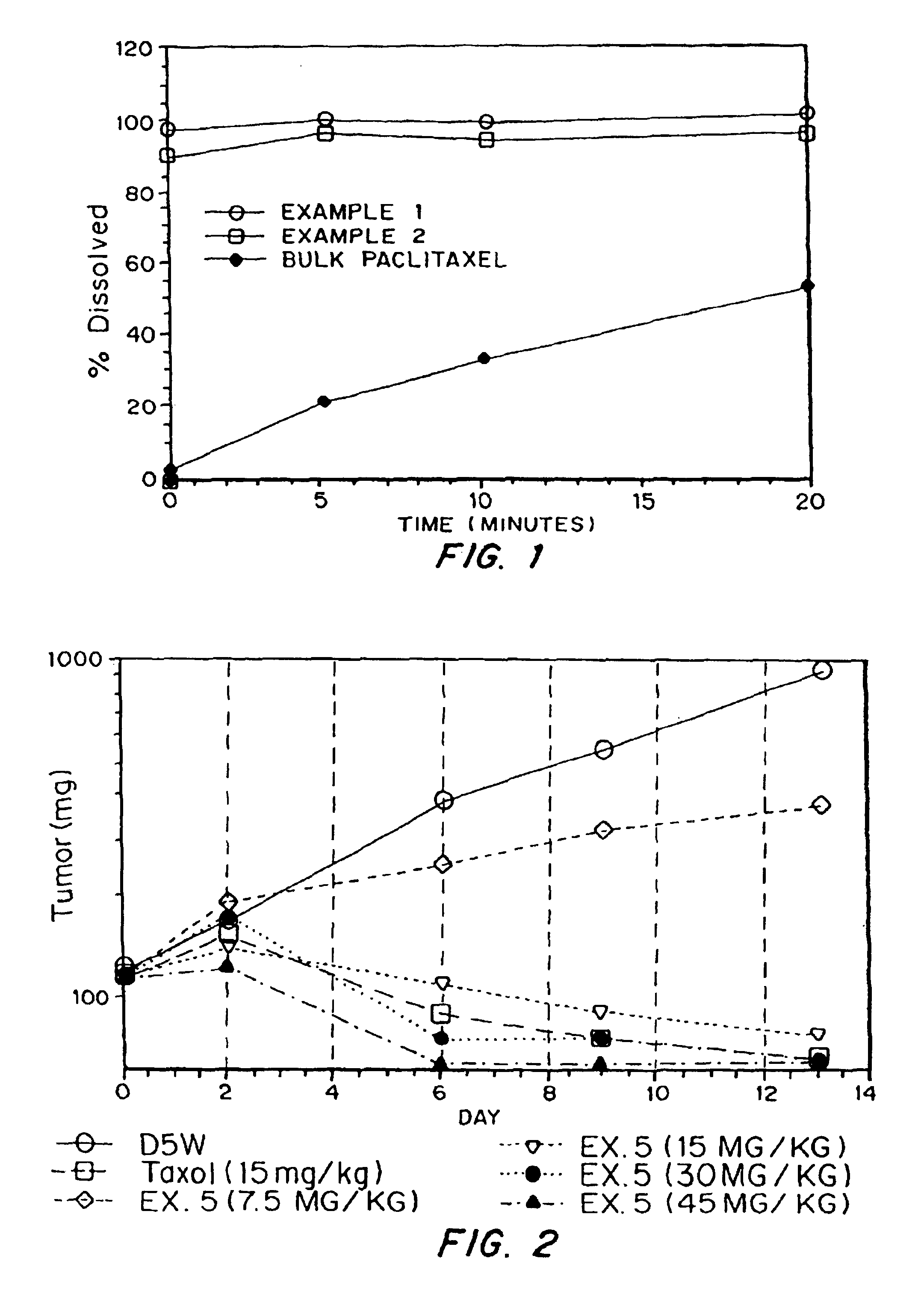
In Vitro Dissolution of Porous Paclitaxel Matrices
[0066]The in vitro dissolution rates of the powders produced in Examples 1-2 were compared to the dissolution rates of the non-formulated paclitaxel.
Analytical Methods
[0067]Studies were conducted in PBS containing 0.08% TWEEN™ 80 (T80 / PBS). T80 / PBS (10 mL) was added to an appropriate amount of material being tested to contain 5 mg of paclitaxel in a 15 mL polypropylene conical tube, and the suspension was vortexed for 3-4 minutes. The suspension (0.25 mL) was then added to 250 mL of T80 / PBS in a 600 mL glass beaker for dissolution analysis. All dissolution studies were conducted using overhead mixing. The mixer used was an IKARW16 Basic Mixer with a R1342 impeller shaft running at stirring rate 5. Samples were removed via pipette, filtered through 0.22 micron CA syringe filter, and then analyzed. Dissolution curves are presented as percent of complete dissolution.
[0068]HPLC analysis was performed directly on the filtered aqueous solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| TAP density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com