Reversal of general anesthesia by administration of methylphenidate, amphetamine, modafinil, amantadine, and/or caffeine
a general anesthesia and reversal technology, applied in the direction of diagnostic recording/measuring, drug composition, biocide, etc., can solve the problems of anesthesia-induced loss of consciousness, unpredictable timing of emergence, pharmacokinetics and pharmacodynamics, etc., to facilitate emergence of the subject, reduce or eliminate the effect of delirium, and restore mobility or consciousness in the subj
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
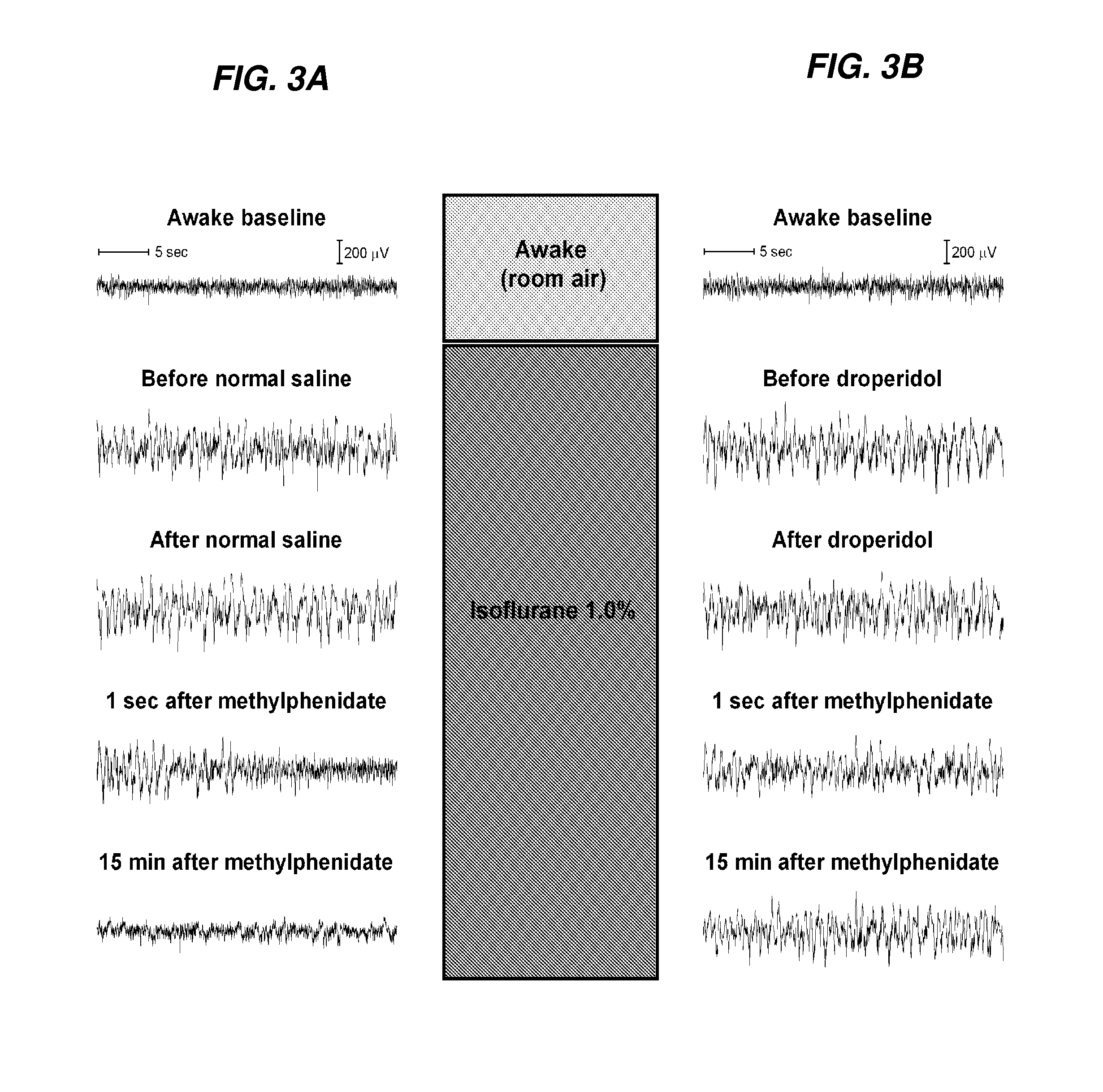
[0225]The inventors herein assessed if methylphenidate (MPH) induces emergence from isoflurane anesthesia. The inventors first tested whether methylphenidate affects time to emergence from a standardized general anesthetic with isoflurane. The inventors then assessed two possible mechanisms by which methylphenidate may act: (1) increased arousal, or (2) increased minute ventilation. To test for increased arousal, the inventors performed experiments to assess whether methylphenidate induces restoration of righting under continuous isoflurane anesthesia. The inventors also performed spectral analysis of electroencephalogram recordings to assess changes induced by methylphenidate during continuous isoflurane anesthesia. To test for increased minute ventilation, the inventors obtained plethysmography and arterial blood gas data to analyze changes in respiratory status induced by methylphenidate.
[0226]Thus, one aspect of the present invention is based on the inventors discovery that in a...
example 2
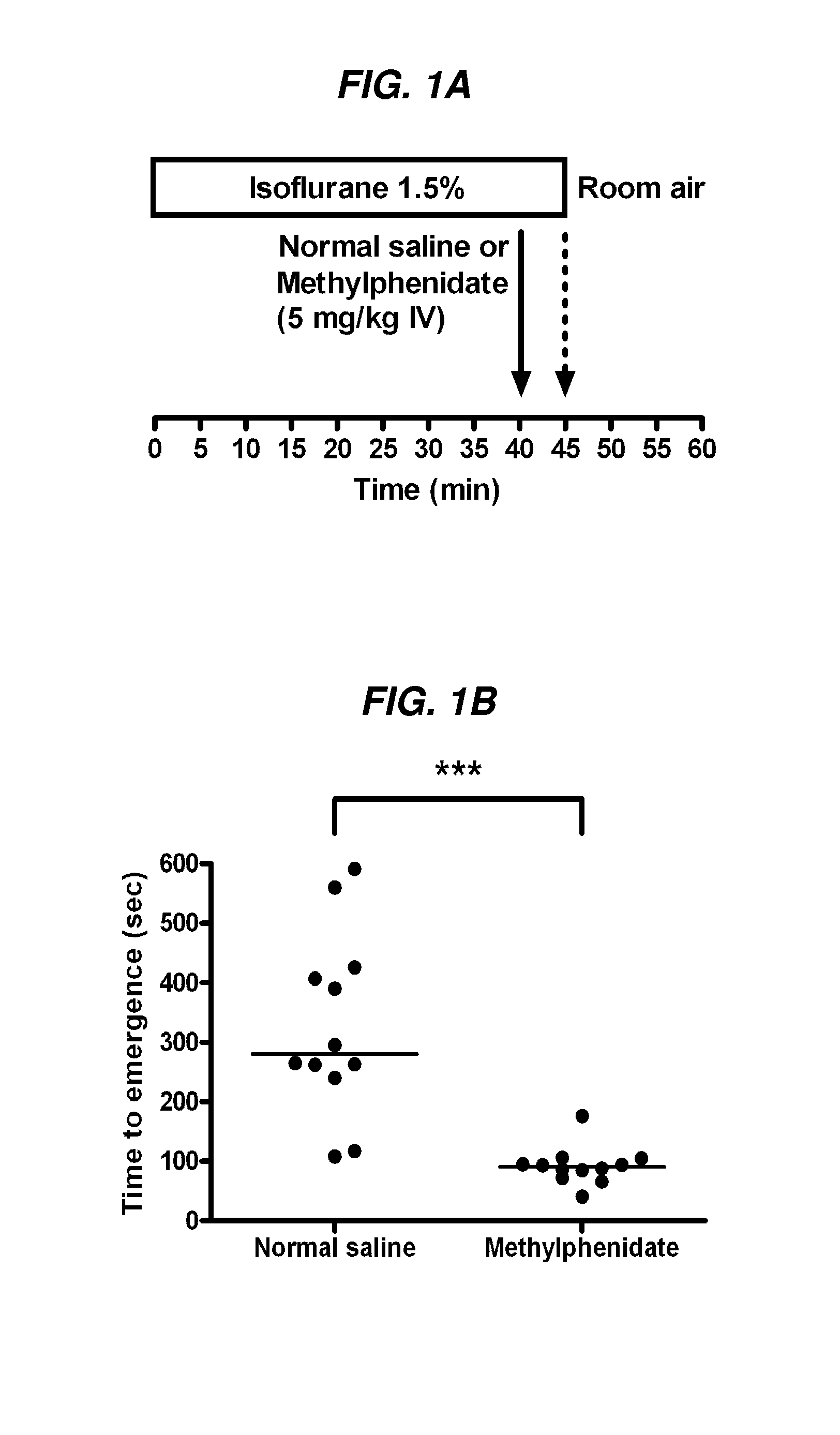
[0229]Methylphenidate Hastens Time to Emergence from a Standardized Isoflurane Anesthetic.
[0230]FIG. 1A provides a schematic of the protocol for this experiment. As shown in FIG. 1B, the median time to emergence for animals that received normal saline was 280 seconds (n=12), versus 91 seconds (n=12) for animals that received methylphenidate (5 mg / kg IV). The median difference in time to emergence between these two groups was 200 seconds with a 95% confidence interval computed using the Mann-Whitney test of [155, 331] seconds. This median difference was statistically significant (p<0.0001, two-sided Mann-Whitney test). The % recovery of consciousness in rats of was faster for D-MGH than L-MPH, as shown in FIG. 7.
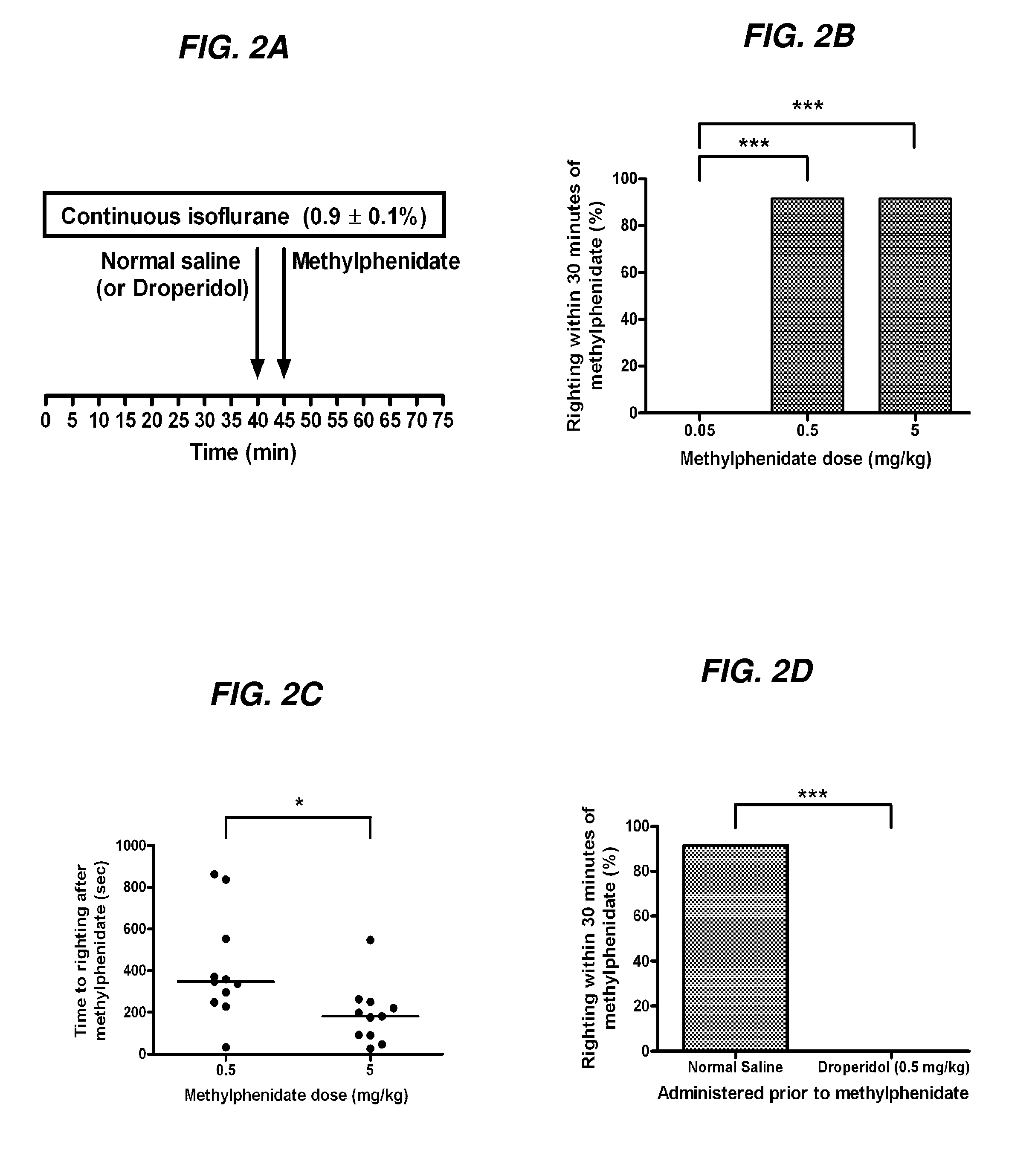
[0231]Methylphenidate Induces Emergence During Continuous Inhalation of Isoflurane
[0232]To assess if methylphenidate increases arousal, the inventors performed the following experiments during continuous inhalation of isoflurane. Because isoflurane was not discontinued, any e...
example 3
[0233]Droperidol Inhibits Methylphenidate-Induced Emergence Behavior
[0234]In a group of animals (n=6) continuously inhaling isoflurane at a dose sufficient to maintain loss of righting as above, the protocol illustrated in FIG. 2A was repeated with the exception that droperidol (0.5 mg / kg IV) was administered in place of normal saline. None of the animals exhibited purposeful movement in response to the administration of droperidol or subsequent removal of the temperature probe. Five minutes after droperidol, the highest dose of methylphenidate used in this study (5 mg / kg) was administered. These animals generally exhibited no purposeful movement after methylphenidate administration, although some sluggish limb movements were occasionally observed. None of these animals had return of righting, compared to the 11 of 12 animals that had return of righting after receiving normal saline prior to the same dose of methylphenidate (FIG. 2D). The 95% Bayesian confidence interval for the dif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| frequencies | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com